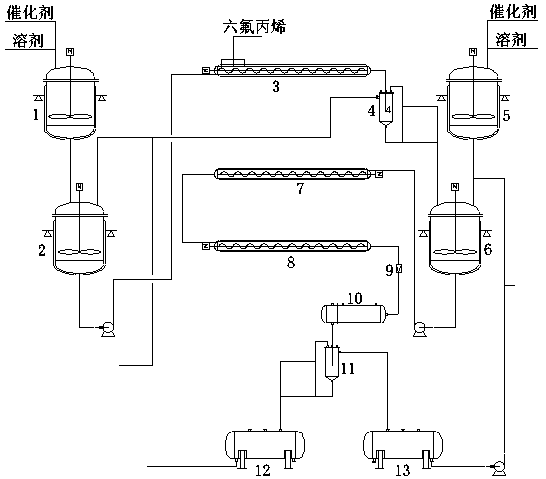
A kind of industrialized production method and production device of perfluoro-2-methyl-2-pentene
A production method and methyl technology, applied in the field of organic fluorine chemical preparation, can solve problems such as long reaction period, achieve the effects of fast reaction speed, improve equipment use efficiency, and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Continuous dimerization synthesis of perfluoro-4-methyl-2-pentene proceeds in the following steps:
[0027]Add 12.2kg of anhydrous potassium fluoride, 15.8kg of 18-crown-6, and 200kg of acetonitrile into the 500L catalyst configuration kettle 1. Turn on the stirring to make the catalyst evenly mix and dissolve into the solvent. Open the bottom valve of the catalyst configuration tank, and measure and put 100 kg of catalyst-solvent system (hereinafter referred to as "system") into the pre-cooling tank 2. Turn on the pre-cooling tank to stir, and open the inlet and outlet valves of the refrigerant. The system was cooled to 10 °C. Open the bottom valve of the pre-cooling tank, turn on the metering pump, open the outlet valve of dimerization tubular reactor 3, meter the system at a rate of 300kg / h into the reactor (residence time 3min), and open the dimerization tubular reactor Stir. After the system is full of the reactor, slowly open the valve of the hexafluoropropyle...
Embodiment 2
[0030] Continuous dimerization synthesis of perfluoro-4-methyl-2-pentene proceeds in the following steps:
[0031] The same method was operated as in Example 1, except that the dimerization reaction temperature was 20°C.
[0032] Results: A total of 121.1kg of HFP (content 99.9493%) was introduced to obtain 119.9kg of dimers, of which HFP residue was 0.014%, the total content of dimers was 98.42%, and the total content of multimers was 1.27%. The yield was 97.49%
Embodiment 3
[0034] Continuous dimerization synthesis of perfluoro-4-methyl-2-pentene proceeds in the following steps:
[0035] The same method was operated as in Example 1, except that the dimerization reaction temperature was 0°C.
[0036] Results: A total of 121.7kg of HFP (content 99.9493%) was introduced to obtain 120.3kg of dimers, of which HFP residue was 0.14%, the total content of dimers was 99.02%, and the total content of multimers was 0.42%. The yield was 97.93%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com