Method for effectively removing nitrate in underground water by using Zn/Cu/Ti multi-metal nanoelectrode
A nano-electrode and nitrate technology, applied in water/sludge/sewage treatment, sterilization/microdynamic water/sewage treatment, polluted groundwater/leachate treatment, etc., can solve the problem of multi-metal nano-electrode removal of nitric acid in water Salt has not been reported and other problems, to achieve the effect of low cost, convenient operation and simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
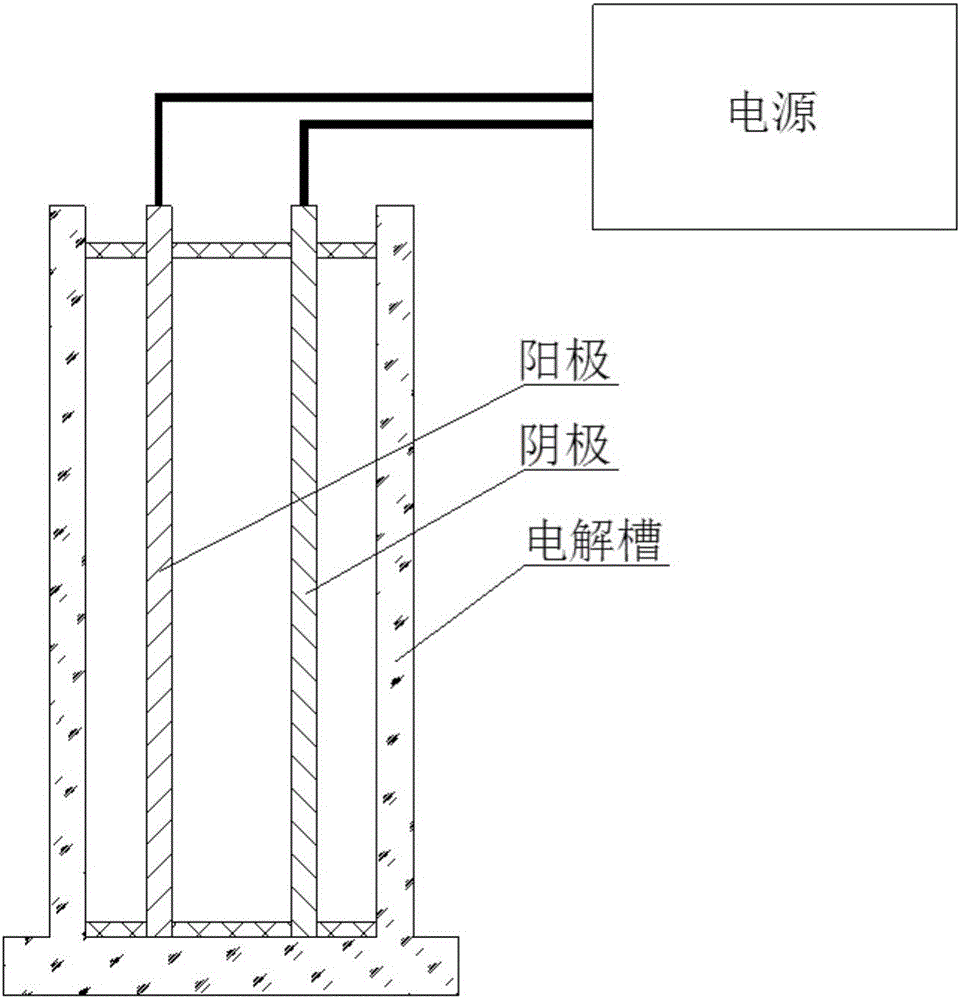
[0056] Such as figure 1 As shown, the electrolytic cell is a cylindrical water tank made of polyethylene material, a DC voltage stabilizer is used as the power supply, the effective voltage is 0-100V, and the effective current is 0-10A.
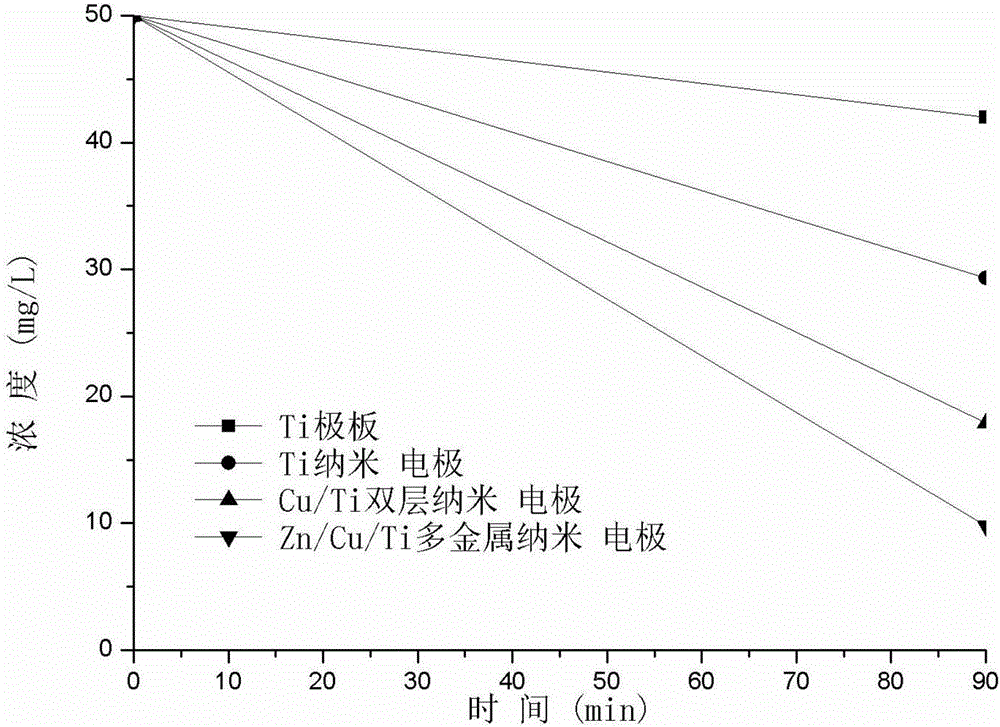
[0057] In the experiment, the nitrate-contaminated water (NO 3 - -N, 50mg / L; Na 2 SO 4 , 0.5g / L) 100mL into the electrolytic cell, turn on the power, adjust the current, so that the current is 0.75A for electrolysis of nitrate-contaminated water. The size of the cathode and anode plates are both 10×2.5cm, and the effective area of the plates is 20.0cm 2 , the anodes all use Pt electrodes, ① the cathode used is Ti cathode, and the concentration of nitrate nitrogen drops from 50.0mg / L to 42.0mg / L after 90 minutes of reaction; ② the cathode uses Ti nano-electrodes made of graphite electrodes as auxiliary electrodes (Ti-1), then the concentration of nitrate nitrogen drops from 50.0mg / L to 29.3mg / L after 90 minutes of reaction, and the remo...
Embodiment 2
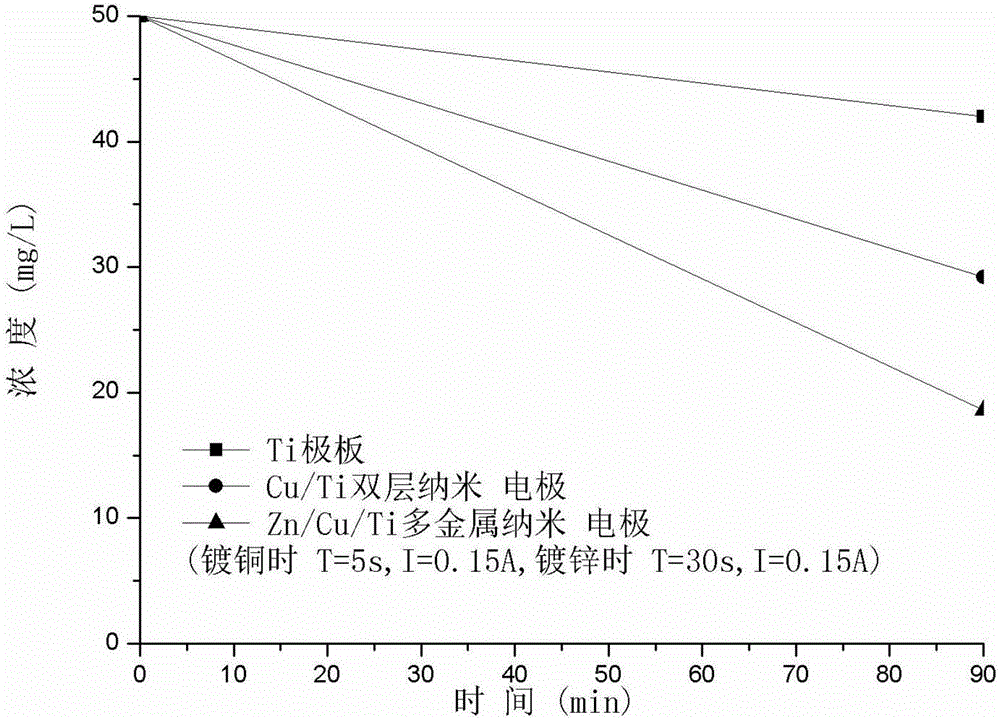
[0059] The electrolyzer used in the experiment and the method of using Zn / Cu / Ti multi-metal nano-electrode to remove nitrate are as example 1, the difference is that when making Zn / Cu / Ti multi-metal nano-electrode, the copper plating time is 5 seconds, and when copper plating The current used is 0.15A, the galvanizing time is 30 seconds, and the current used during galvanizing is 0.15A. The concentration of nitrate nitrogen drops from 50.0mg / L to 18.6mg / L after 90 minutes of reaction using the multi-metal nanoelectrode. The removal rate increased by 392% compared with the use of Ti electrodes, and by 21.2% compared with the use of Cu / Ti double-layer nano-electrodes, which greatly improved the removal efficiency of nitrate. The results are as follows image 3 shown.
Embodiment 3
[0061] The electrolytic cell used in the experiment and the method of using Zn / Cu / Ti multi-metal nano-electrode to remove nitrate are as in Example 1, the difference is that when making Zn / Cu / Ti multi-metal nano-electrode, first galvanize, and the galvanizing time is 10 seconds , the current used is 0.3A, after copper plating, the copper plating time is 10 seconds, the current used is 0.15A, and the concentration of nitrate nitrogen drops from 50.0mg / L to 24.8mg / L after 90 minutes of reaction using the multi-metal nanoelectrode L, the removal rate is 315% higher than that of using Ti electrode, and 13.2% higher than that of using Cu / Ti double-layer nanoelectrode, which greatly improves the removal efficiency of nitrate. The results are as follows Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com