Method for preparing dimethylamino ethyl bilobalide B by using micro reaction device
A technology of dimethylaminoethyl and micro-reaction devices, applied in the direction of organic chemistry, can solve the problems of high reaction cost, low conversion rate of raw materials, long reaction time, etc., and achieve high conversion rate, simple and efficient process, and stable product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
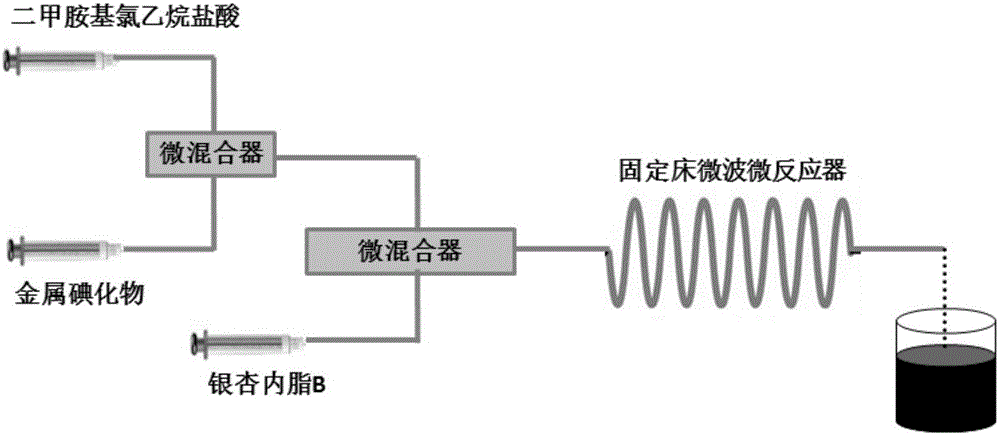
[0024] The acetonitrile solution of dimethylaminoethyl chloride hydrochloride (concentration: 0.01g / mL, flow rate: 1mL / min) and the aqueous solution of potassium iodide (concentration: 0.001g / mL, flow rate: 0.1mL / min) were pumped into micro Mixer, then the acetonitrile solution of ginkgolide B (concentration is 0.01g / mL, flow rate is 1mL / min) and the above-mentioned micro-mixer discharge pump into the fixed-bed microwave microreactor filled with potassium carbonate, the ginkgo The mol ratio of lipid B and dimethylaminoethyl chloride ethyl chloride hydrochloride is 1:1, the mol ratio of potassium iodide and ginkgolide B is 0.2:1, and the mol ratio of potassium carbonate and ginkgolide B is 1.5:1, Keep the reaction residence time for 0.5 min, react at 50°C, filter the crude product, concentrate the filtrate, and recrystallize the obtained solid with methanol to obtain dimethylaminoethyl ginkgolide B with a yield of 85%.
Embodiment 2
[0026] Acetonitrile solution of dimethylaminoethyl chloride hydrochloride (concentration: 0.03 g / mL, flow rate: 2 mL / min) and aqueous solution of potassium iodide (concentration: 0.0025 g / mL, flow rate: 1 mL / min) were pumped into micro-mixing device, then the acetonitrile solution of ginkgolide B (concentration is 0.04g / mL, flow rate is 5mL / min) and the above-mentioned micro-mixer discharge pump into the fixed-bed microwave microreactor filled with cesium carbonate, ginkgolide The mol ratio of B and dimethylaminoethyl chloride ethane hydrochloride is 1:2, and the mol ratio of potassium iodide and ginkgolide B is 0.5:1, and the mol ratio of cesium carbonate and ginkgolide B is 1.8:1, keeps The reaction residence time was 0.75 min, and the reaction was carried out at 60°C. The crude product was filtered, the filtrate was concentrated, and the obtained solid was recrystallized with methanol to obtain dimethylaminoethyl ginkgolide B with a yield of 92%.
Embodiment 3
[0028] Acetonitrile solution of dimethylaminoethyl chloride hydrochloride (concentration: 0.04g / mL, flow rate: 3mL / min) and potassium iodide aqueous solution (concentration: 0.0025g / mL, flow rate: 0.37mL / min) were pumped into micro Mixer, then the acetonitrile solution of ginkgolide B (concentration is 0.03g / mL, flow rate is 2.9mL / min) and the above-mentioned micro-mixer discharge pump into the fixed-bed microwave microreactor filled with cesium carbonate, ginkgo biloba The molar ratio of lactone B to dimethylaminoethylene chloride hydrochloride is 1:2, the molar ratio of sodium iodide to ginkgolide B is 0.5:1, and the molar ratio of cesium carbonate to ginkgolide B is 1.8 : 1, keep the reaction residence time 0.75min, react at 60°C, filter the crude product, concentrate the filtrate, recrystallize the gained solid with methanol, obtain dimethylaminoethyl ginkgolide B, and the productive rate is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com