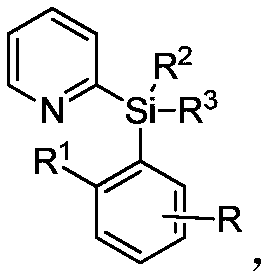
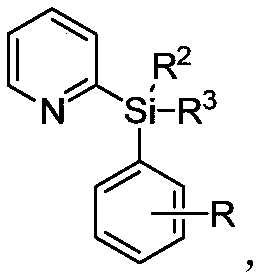
A kind of preparation method of alkyl modified pyridyl aryl silane compound
A pyridyl aryl silane and compound technology, which is applied in the field of preparation of pyridyl aryl silane compounds, can solve the problems of complicated operation, harsh conditions, and narrow substrate applicability, and achieve reduction of reaction waste, mild reaction conditions, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of butyl-modified pyridylarylsilane compounds by palladium-catalyzed reaction of pyridylarylsilane with butylboronic acid:
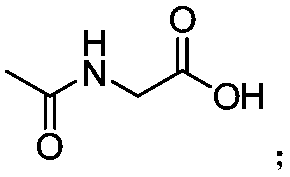
[0035] In the 35mL reaction tube, add a stirrer, 4.5mg Pd(OAc) 2 (10mol%), corresponding pyridylarylsilane (0.2mmol), 30.6mg butylboronic acid (0.6mmol), 4.6mg Ac-Gly-OH (20mol%), 21.8mg BQ (1.2equiv), 110.2mg Ag 2 CO 3 (2.0 equiv), 16.8 mg NaHCO 3 (1.0 equiv) and 1.0 mL THF. Then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 60°C for 48 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Purify and isolate the corresponding butylated product through silica gel plate, weigh ...
Embodiment 2
[0122] Palladium-Catalyzed Alkylation of Pyridylphenyldiisopropylsilane:
[0123] In the 35mL reaction tube, add a stirrer, 4.5mg Pd(OAc) 2(10mol%), corresponding pyridylarylsilane (0.2mmol), alkylboronic acid (0.6mmol), 4.6mg Ac-Gly-OH (20mol%), 21.8mg BQ (1.2equiv), 110.2mg Ag 2 CO 3 (2.0 equiv), 16.8 mg NaHCO 3 (1.0 equiv) and 1.0 mL THF. Then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 60°C for 48 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Purify and isolate the corresponding alkylated product through silica gel plate, weigh to determine the yield, and use NMR and HRMS for qualitative detection.
...
Embodiment 3
[0175] Pyridyl aryl silane, butyl boronic acid, glycine whose nitrogen atom is protected by acetyl group, AgO, KHCO 3 , Benzoquinone, Pd(TFA) 2 Mix at a molar ratio of 1:1:0.02:1:1:0.5:0.01, dissolve in the solvent tert-amyl alcohol, then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 30°C for reaction 48h. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Purify and isolate the corresponding butylated product by silica gel plate, weigh and calculate the yield. The final product is:
[0176]
[0177] The yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com