Biological preparation method of typhoid glycoprotein and application thereof
A protein and pili protein technology, applied in the field of biological preparation of typhoid glycoprotein, can solve the problem of low specificity of polysaccharides, achieve the effects of improving uniformity, reducing costs, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1. Preparation of Salmonella typhi O-polysaccharide-recombinant EPA fusion protein and vaccine thereof by one-step biological cross-linking method
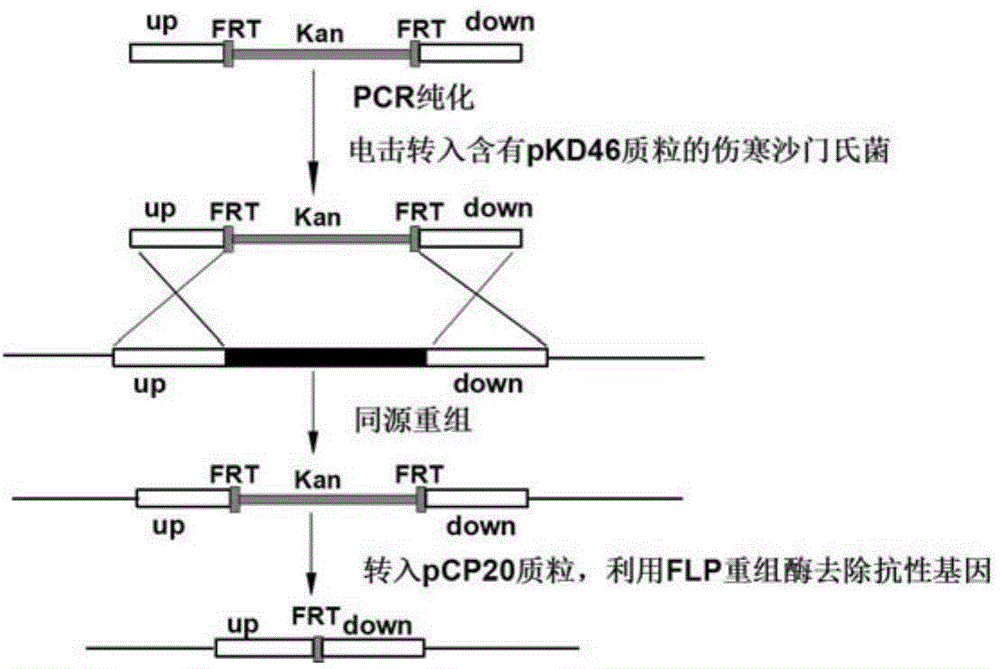
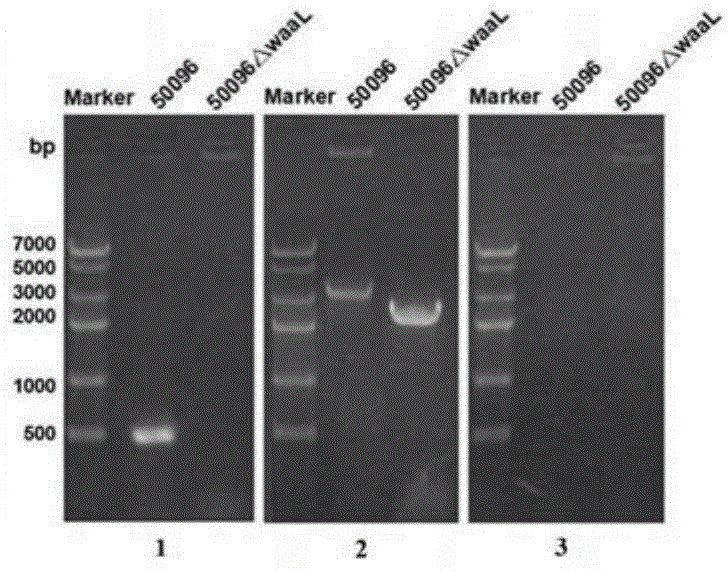
[0074] 1. Preparation of Salmonella typhi deficient in O-antigen ligase gene waaL
[0075] (1) Preparation of linear targeting DNA fragments
[0076] 1. Design of PCR primers
[0077] According to the O-antigen ligase gene waaL (Genbank No. 3925131-3926345 of GI: 29139723) listed in the complete genome sequence of Salmonella typhi on NCBI (NC_004631.1) and its upstream and downstream sequences, the upstream of the waaL gene ( 5' end) and downstream (3' end) respectively design a pair of primers, namely 96waaLu1 / 96waaLu2 and 96waaLd1 / 96waaLd2. For the convenience of operation, restriction enzyme cutting sites BamH Ⅰ and Sal Ⅰ will be added to the end of the primer of the upstream homology arm up, and restriction enzyme cutting sites Hind III and Xho I will be added to the end of the primer of the downstream homolog...
Embodiment 2
[0157] Example 2, One-step biological cross-linking method to prepare Salmonella typhi O-polysaccharide-PilE protein
[0158] 1. Preparation of Salmonella typhi deficient in O-antigen ligase gene waaL
[0159] The steps are the same as Step 1 in Example 1.
[0160] 2. Molecular and phenotypic identification of Salmonella typhi defective in lipopolysaccharide synthesis
[0161] The steps are the same as Step 2 in Example 1.
[0162] 3. Preparation of Salmonella typhi O-polysaccharide-PilE protein by one-step biological cross-linking method
[0163] (1) Construction of glycosyl-engineered Salmonella typhi
[0164] The vectors pMMB66EH-pilE and pETtac28-pglL were electroporated to transform the host bacteria 50096ΔwaaL successively, and the double-antibody LB plate containing the final concentration of 50 μg / mL kanamycin and 100 μg / mL ampicillin was coated, and the grown clones were Typhoid glycosylated Engineering bacteria 50096ΔwaaL / pMMB66EH-pilE / pETtac28-pglL.
[0165] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com