Immunologic adjuvant composition and preparation method thereof
A technology of immune adjuvant and composition, which is applied in the direction of biochemical equipment and methods, microorganisms, antiviral agents, etc., and can solve the problems of unsatisfactory immune effect and inability to protect animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the preparation of the linear alkyl ester of acrylic acid
[0047] Dissolve 1 g of Carbomer 907 in 50 ml of the corresponding n-octanol, and heat the solution to 135°C. 50 microliters of sulfuric acid was added and the reaction mixture was maintained at 135°C. The reaction was stopped by rapidly cooling the reaction mixture and adding 1 volume of distilled water. Then, the pH of the solution was adjusted to pH=6, and the pressure was reduced at 80° C. (10 -6 ba) Evaporation of the solvent. The product thus obtained was dissolved in distilled water, dialyzed against distilled water, and then lyophilized to give a product named alkylcarbomer 907(8).
Embodiment 2
[0048] Embodiment 2, the preparation of mycobacterium phlei culture
[0049] Mycobacterium phlei strains were cultured in a fermenter at 37°C with aeration, the pH value was 7.0, and the culture time was 4 days. The medium formula: 1g of yeast extract powder, 3ml of concentrated wort juice, 25g of glucose, Diammonium hydrogen phosphate 3g, dipotassium hydrogen phosphate 0.8g, add water for injection to 1000ml. After inactivating the fermentation broth with formaldehyde solution (0.1%), centrifuge and concentrate, discard the supernatant, take the lower layer of bacteria and resuspend 3 times with an appropriate amount of normal saline, centrifuge to remove impurities, measure water, and calculate the weight of dry bacteria. After homogenizing the physiological saline, perform high-pressure homogenization, and then freeze-dry to obtain the culture of Mycobacterium phlei.
example 3
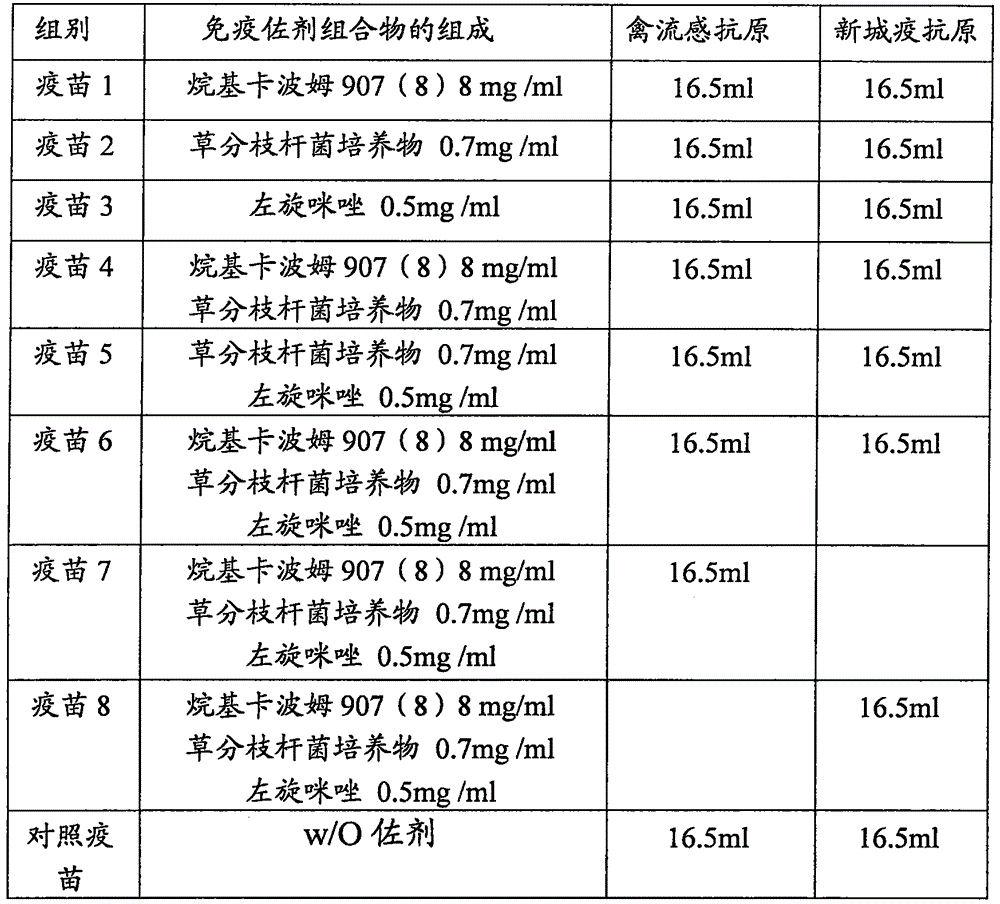
[0050] Example 3: Vaccine Composition Preparation
[0051] 3.1 Preparation of antigens: Prepare avian influenza antigens and Newcastle disease antigens according to Chinese patent CN101607084, and the virus content before inactivation is 10 8.20 (EID 50 / 0.1ml), the antigen prepared by this method does not constitute a limitation of the present invention, those skilled in the art should understand that adopting the avian influenza and Newcastle disease antigen prepared by other strains or adopting from the commercialized product can still realize the object of the present invention.
[0052] 3.2 Preparation of the vaccine composition: add the alkylcarbomer 907 (8) prepared in Example 1, the Mycobacterium phlei culture prepared in Example 2 and levamisole according to the addition amount in Table 1, and add 3.1 to prepare the inactivated For bird flu and / or Newcastle disease antigen, adjust the volume to 100ml with PBS solution with pH=7.2. Stir at room temperature for 30 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com