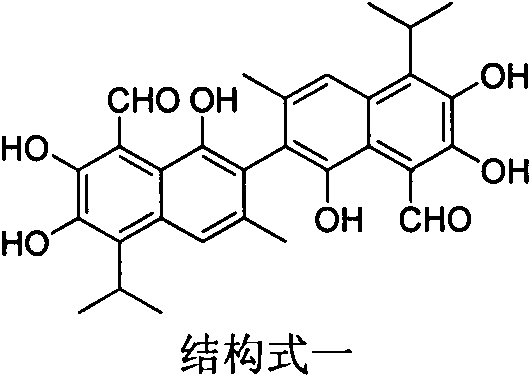
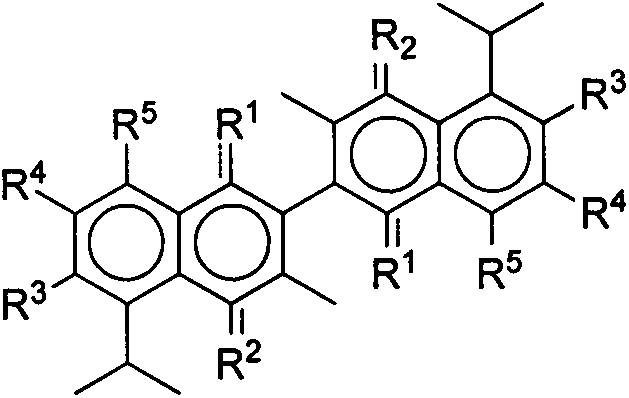
Gossypol derivatives and preparation thereof, application of gossypol derivatives in pesticide and anti-cancer activity
A kind of derivative, gossypol technology, applied in gossypol derivatives and their preparation, in the field of pesticide application and anticancer activity, to achieve non-target biosafety, good environmental compatibility, and significant anti-plant virus activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Gossypol-methylamine Schiff base (I a -1) Synthesis
[0043]
[0044] In a 100 mL round-bottom flask, add 0.50 g (0.86 mmol) of gossypol acetate and 40 mL of absolute ethanol, and then add 0.05 g (1.73 mmol) of methylamine after the solution is completely dissolved. The reaction solution was heated to reflux for 5 hours. After being naturally cooled to room temperature, the reaction solution was filtered to obtain a bright yellow solid. Yield, 96%; melting point: 258-259°C; 1 H NMR(400MHz, DMSO-d 6 )δ13.04(dd, J=13.0, 4.6Hz, 2H), 9.73(d, J=13.0Hz, 2H), 8.39(s, 2H), 7.84(s, 2H), 7.45(s, 2H), 3.75-3.66(m, 2H), 3.27(d, J=4.6Hz, 6H), 1.93(s, 6H), 1.43(t, J=6.4Hz, 12H); 13 C NMR(100MHz, DMSO-d 6 )δ171.47,163.60,149.58,146.22,131.07,126.74,126.13,119.96,116.44,115.81,103.08,36.84,26.46,20.34,20.31,20.17; HRMS(ESI)m / z calcd for C 32 H 37 N 2 O 6 [M+H] + 545.2646, found 545.2645.
Embodiment 2
[0045] Example 2: Gossypol fatty amine Schiff base derivative I a -2-I a -16, I a -23, gossypol oxime, hydrazone Schiff base derivative I b -3-I b Synthesis of -7: Completed by repeating the method of Example 1
[0046] Compound I a -2: The specific operation is carried out according to operation step A. Yellow solid; yield, 96.1%; melting point 240-241°C; 1 H NMR(400MHz, CDCl 3 )δ13.38-13.35(m, 2H), 9.64(d, J=12.7Hz, 2H), 8.01(s, 2H), 7.60(s, 2H), 5.57(s, 2H), 3.71-3.75(m , 2H), 3.52-3.43 (m, 4H), 2.12 (s, 6H), 1.69-1.78 (m, 4H), 1.50-1.55 (m, 12H), 1.00 (t, J = 7.2 Hz, 6H); 13 C NMR(100MHz, CDCl 3 )δ172.44,163.03,148.91,147.19,131.70,128.89,127.15,118.16,115.63,114.72,103.02,52.50,27.42,23.91,20.40,20.34,20.07,11.14; HRMS(ESI)m / z calcd for C 36 H 45 N 2 O 6 [M+H] + 601.3272, found 601.3271.
[0047] Compound I a -3: The specific operation is carried out according to operation step A. Bright yellow solid; Yield, 89.6%; Melting point: 234-235°C; 1 H NMR(400MHz, CDCl 3 )δ13.41-13....
Embodiment 3
[0067] Example 3: Gossypol-2-sulfonate sodium ethylamine Schiff base (I a -18) Synthesis:
[0068]
[0069] In a 100mL round-bottomed flask, add 0.069g (1.73mmol) of sodium hydroxide, 40mL of absolute ethanol, and 0.22g (1.73mmol) of taurine. Heat at reflux for 1h to generate sodium salt (pH value is about 8), then add 0.50 g (0.86 mmol) of gossypol acetate, heating for 5 hours, cooling to room temperature, suction filtration, recrystallization with isopropanol and methanol to obtain the target compound. Yield, 94.0%; melting point>300℃; 1 H NMR(400MHz, DMSO-d 6 )δ13.07-12.95(m, 2H), 10.03(d, J=11.2Hz, 2H), 8.51(s, 2H), 7.28(s, 2H), 3.78(d, J=3.8Hz, 4H), 3.72-3.62 (m, 2H), 2.82 (t, J=5.5Hz, 4H), 1.95 (s, 6H), 1.46-1.41 (m, 12H). 1 H NMR (400MHz, CD 3 OD)δ9.90(s, 2H), 7.56(s, 2H), 3.99(t, J=6.3Hz, 4H), 3.82-3.74(m, 2H), 3.18(t, J=6.3Hz, 4H) , 2.05 (s, 6H), 1.54-1.50 (m, 12H). 13 C NMR(100MHz, DMSO-d 6 )δ171.33, 161.82, 152.46, 146.34, 131.01, 126.79, 126.29, 121.87, 116.34, 114.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com