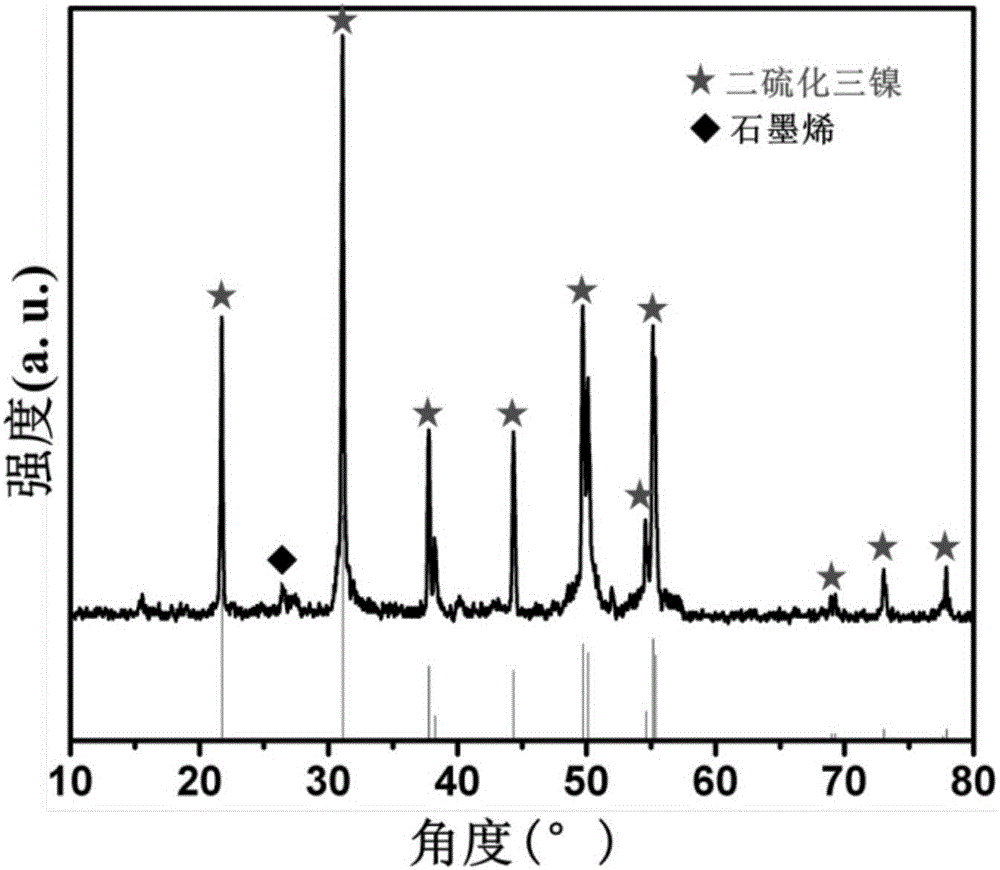
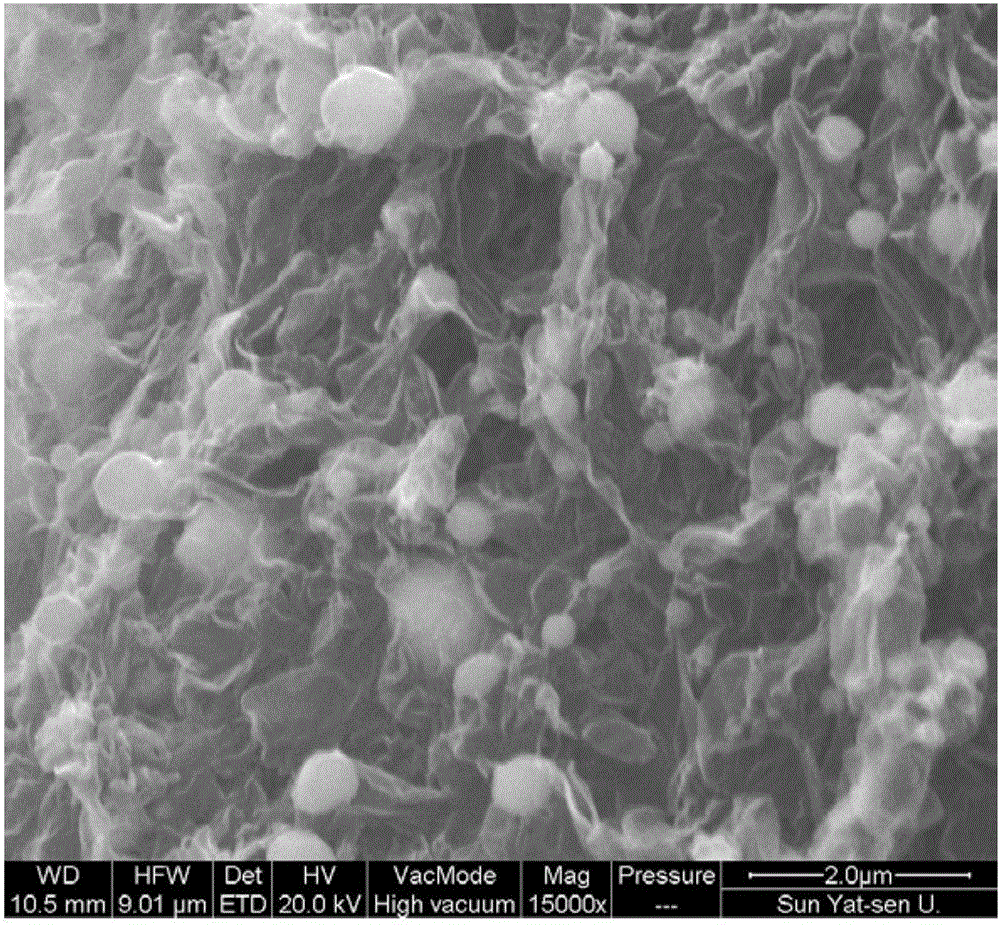
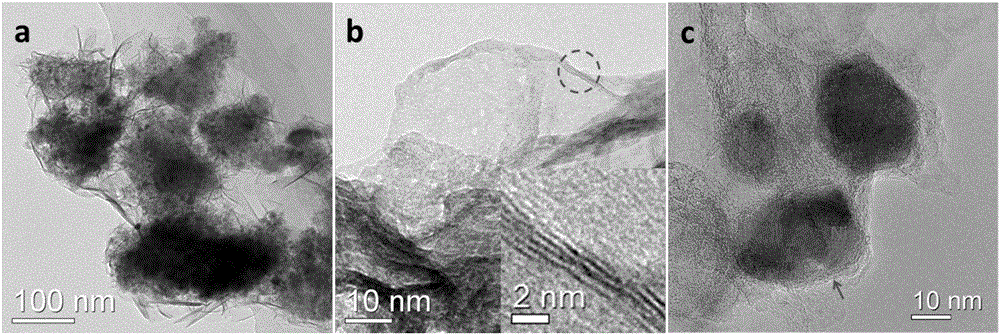
Preparation method for nitrogen-doped graphene-coated nickel sulfide composite electrode material
A technology of nitrogen-doped graphene and composite electrodes, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of difficult control and batch preparation, harsh reaction conditions, uneven distribution, etc., and achieve low cost and convenient mass production , the reaction conditions and the simple effect of the device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Soak 200g of nitrogen- and sulfur-containing thiourea resin in 1.1L of sulfuric acid solution with a concentration of 0.5mol / L and stir for 6h, then filter it, wash it with deionized water until the pH is neutral, and then dry it at 80°C for 24h. After drying, pulverize with a pulverizer for use;
[0028] (2) Gained thiourea resin 3.0g after pulverizing in the step (1) is spread on the porcelain boat, then covers the foamed nickel on the porcelain boat;
[0029] (3) Transfer the porcelain boat covered with nickel foam obtained in step (2) into a high-temperature furnace, and heat it to 800° C. at a heating rate of 3° C. / min under nitrogen protection and keep it warm for 0.5 hours. Simultaneously, the sulfur containing nitrogen and sulfur Thermal decomposition of urea resin provides gaseous carbon source, nitrogen source and sulfur source to react on the surface of nickel foam, and the substance produced by the reaction is on the surface of nickel foam. After the rea...
Embodiment 2
[0031] (1) Soak 200g of nitrogen- and sulfur-containing mercaptoamine type chelating resin with 0.8L concentration of 1.0mol / L hydrochloric acid solution and stir for 8h, filter it, and wash it with deionized water until the pH is neutral, then 80°C Dry for 24 hours, after drying, pulverize with a pulverizer for use;
[0032] (2) get the gained mercaptoamine type chelating resin 4.0g after being pulverized in the step (1) and lay flat on the corundum boat, then cover the nickel sheet on the corundum boat;
[0033] (3) Transfer the corundum boat covered with nickel sheets obtained in step (2) into a high-temperature furnace, and heat it to 850° C. at a heating rate of 5° C. / min under argon protection and keep it warm for 0.75 hours. Simultaneously, nitrogen and sulfur-containing The thermal decomposition of mercaptoamine-type chelating resin provides gaseous carbon source, nitrogen source and sulfur source to react on the surface of the nickel sheet. The substances produced by ...
Embodiment 3
[0035] (1) 200g of nitrogen-containing and sulfur-containing isothiourea chelating resins with 2.0L concentration is that the sulfuric acid solution nitric acid solution of 2.0mol / L soaks and stirs after 6h and filters, and it is neutral to be cleaned to pH with deionized water, then Dry at 80°C for 24 hours, and then pulverize with a pulverizer for later use;
[0036] (2) get the gained isothiourea chelating resin 1.5g after pulverizing in the step (1) and lay flat on the quartz boat, then cover nickel foil on the quartz boat;
[0037] (3) Transfer the quartz boat covered with nickel foil obtained in step (2) into a high-temperature furnace, and heat it to 900° C. at a heating rate of 8° C. / min under nitrogen protection and keep it warm for 1.5 hours. The thermal decomposition of thiourea chelating resin provides gaseous carbon source, nitrogen source and sulfur source to react on the surface of nickel foil, and the substances produced by the reaction are on the surface of ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com