Application of loganin in preparation of drugs or health care products for improving sleep
A technology for improving sleep and improving sleep quality, applied in the application field of medicine or health care products, can solve the problems of improving sleep, not seen, not seen any research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
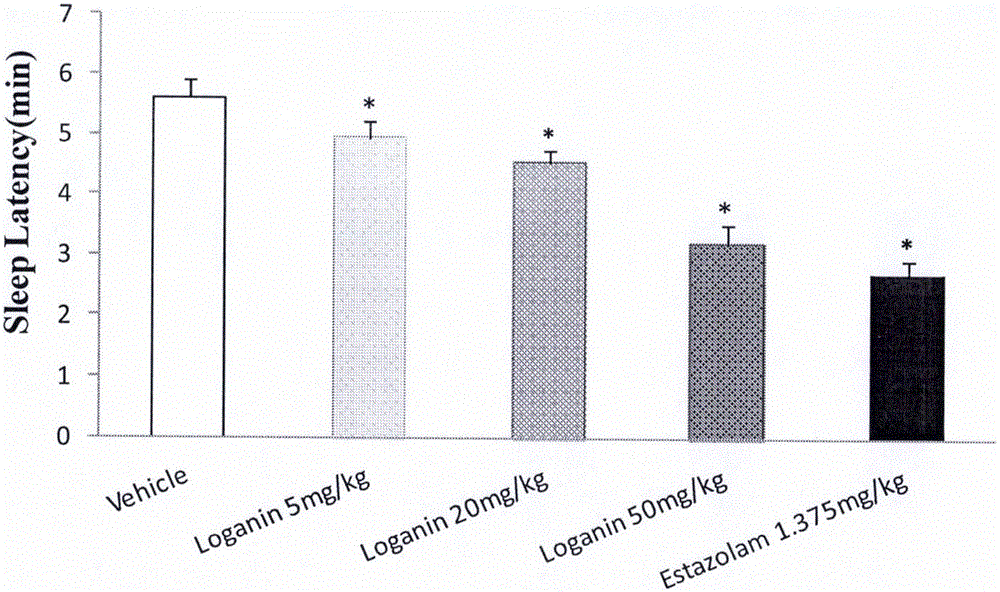
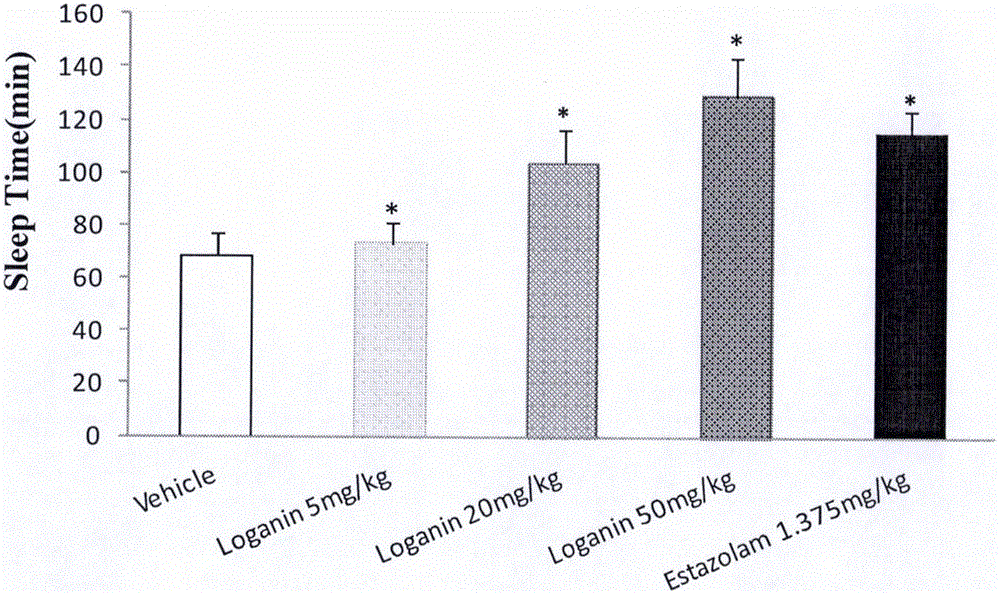
[0043] Example 1 Effect of loganin on sleep time and sleep latency of mice induced by suprathreshold dose of pentobarbital sodium
[0044] Male ICR mice with a body weight of 20-24 g (provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., animal qualification certificate number: SCXK (Beijing) 2012-0001) were randomly divided into blank solvent control groups according to body weight , Estazolam (Estazolam, produced by Shandong Xinyi Co., Ltd., batch number: 150910) 1.375mg / kg group, loganin 5mg / kg group, 20mg / kg group and 50mg / kg group, 15 in every group, fed Stomach administration, and record the administration time, 20min after intraperitoneal injection of suprathreshold dose of pentobarbital sodium (45mg / kg). The disappearance of righting reflex was taken as the time of falling asleep, and the recovery of righting reflex was taken as the time of waking up, and the sleep latency and sleep duration of the mice were recorded.
[0045] The experimental re...
Embodiment 2
[0049] Example 2 Effect of loganin on sleep-onset rate of mice injected intraperitoneally with subthreshold dose of pentobarbital sodium
[0050] Male ICR mice weighing 20-24 g were randomly divided into blank solvent control group, estazolam 1.375 mg / kg group, loganin 5 mg / kg group, 20 mg / kg group and 50 mg / kg group according to body weight. Group, 20 mice in each group, administered by intragastric administration, and recorded the administration time, intraperitoneally injected a subthreshold dose of pentobarbital sodium (22mg / kg) after 20min, observed and recorded the sleeping situation of the mice.
[0051] The experimental results are as shown in Table 2. Loganin 20 and 50 mg / kg orally administered can significantly increase the number of mice falling asleep caused by subthreshold dose pentobarbital sodium (P<0.05). The experimental results show that the horses Qiangside and subthreshold dose of pentobarbital sodium have a synergistic hypnotic effect.
[0052] Table 2 The ...
Embodiment 3
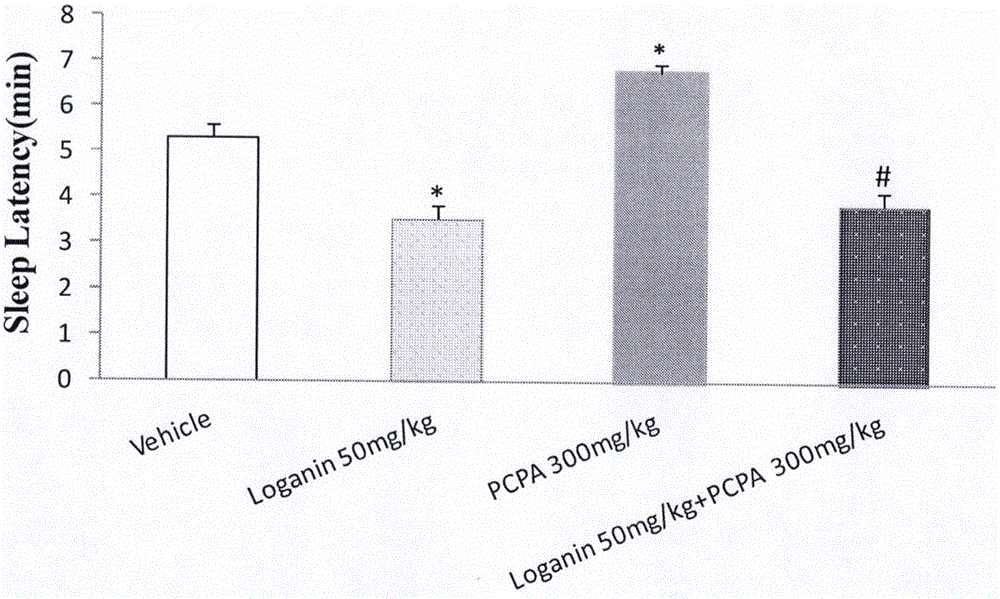
[0055] Example 3 Intervention of loganin on PCPA-induced insomnia model
[0056] Male ICR mice with a body weight of 20-24 g were randomly divided into blank solvent control group, PCPA (p-chlorophenylalanine, purchased from Sigma, 350 mg / kg, i.p.) group, loganin (50 mg / kg, i.g.) group and PCPA (350mg / kg, i.p.)+loganin (50mg / kg, i.g.) group. The administration method is firstly to inject PCPA into the abdominal cavity continuously for 3 days to establish the insomnia model in mice. After 1 hour of intraperitoneal injection of PCPA on the 3rd day, loganin is administered into the stomach, and 20 minutes later, the upper threshold dose of pentobarbital sodium is injected intraperitoneally to reverse the insomnia. The time of falling asleep was defined as the disappearance of the righting reflex, and the time of waking up when the righting reflex returned. The sleep latency and sleep duration of the mice were recorded.
[0057] The experimental results are shown in Table 3. Log...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com