Preparation method and application of Brucella multi-epitope fusion protein vaccine
A fusion protein, brucellosis technology, applied in the field of brucellosis multi-epitope fusion protein antigen, preparation of brucellosis vaccine, can solve problems such as poor immune effect and miscarriage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Design of multi-epitope fusion gene of Brucella and construction of plasmid
[0022] The amino acid sequence of the dominant outer membrane protein of Brucella was retrieved from the Pubmed database, using BepiPred (http: / / www.cbs.dtu.dk / services / Bepipred / ), ABCpred (http: / / www.imtech.res .in / raghava / abcpred / ) and COBEpro (http: / / scratch.proteomics.ics.uci.edu / ) and other software to predict the B cell epitope of Brucella outer membrane protein, using IEDB (http: / / tools.iedb.org / main / tcell / ) The T cell epitope prediction tool on the website predicts T cell epitopes, uses BLASTPanalysis to analyze the similarity of antigenic epitopes, selects the dominant antigenic epitope, and selects the dominant antigenic epitope. Add a linking peptide sequence between them, use DNA Star to optimize the tandem sequence of antigenic epitopes and the type of linking peptide, and select a sequence with good immunogenicity as the amino acid sequence of the target fusion prot...
Embodiment 2
[0027] Example 2 Expression of Brucella rMEP
[0028] The expression host strain Escherichia coli BL21 (DE3) was preserved by the Department of Health Inspection, School of Public Health, Jilin University; the expression vector pET-28b plasmid containing the base sequence of Brucella rMEP vaccine was constructed in Example 1; various molecular Biological reagents were purchased from Biological Reagent Company.
[0029] Transform the pET-28b plasmid obtained in Example 1 into Escherichia coli BL21 (DE3) expression bacteria, heat shock at 42°C for 90s, let it stand on ice for 2 minutes, spread it on an LA plate (containing 30 μg / mL kanamycin), and culture at 37°C overnight. Pick a single colony of the expressed strain and culture it in 4mL LB medium (containing 30μg / mL kanamycin) at 37°C and 220r / min for 18-24h. Take 0.4mL of the culture and add it to 4mL of fresh LB medium (containing 30μg / mL kanamycin) to continue the culture. When the OD of the bacterial solution 600 When ...
Embodiment 3
[0031] Example 3 Purification of Brucella rMEP
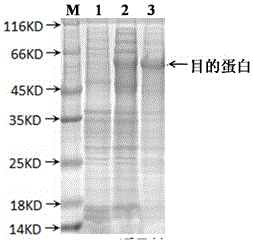
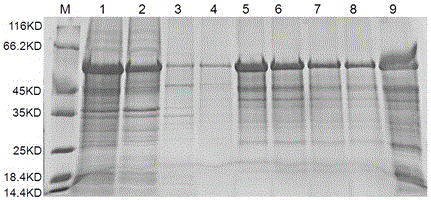
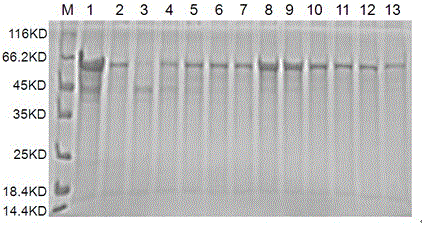
[0032]Get the supernatant after the ultrasonic crushing of above-mentioned embodiment 2, contain Brucella soluble rMEP in the described bacterial supernatant, adopt nickel agarose affinity chromatography and anion exchange chromatography to the Brucella in the supernatant Bacteria-soluble rMEP was purified. The specific purification solution and purification method were prepared and operated according to the instructions of the relevant kit. After the eluted components of the collected purified protein liquid were detected by SDS-PAGE, the high-purity components were dialyzed into PBS. In buffer solution (containing 0.1% SKL, pH = 7.4), dialyze at 4°C overnight, filter and sterilize with a 0.45 μm filter membrane, aliquot and store in a -80°C refrigerator for long-term storage. SDS-PAGE electrophoresis and Western Blot detection were used to detect the purified fusion protein, and the results are shown in the attached Figure 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com