Microenvironment double-response chitosan gene carrier as well as preparation method and application thereof
A dual-response, gene carrier technology, applied in the direction of using the carrier to introduce foreign genetic material, recombinant DNA technology, etc., to achieve the effect of solving the contradiction between gene compression and release, low cytotoxicity, and high gene transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
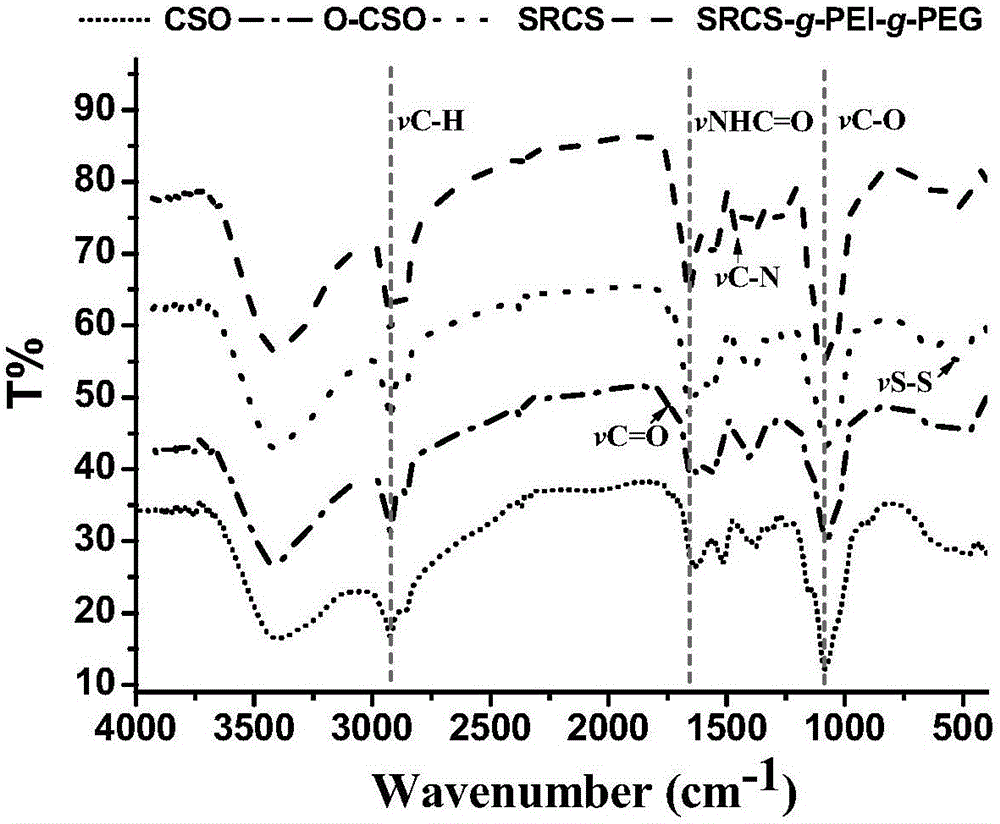
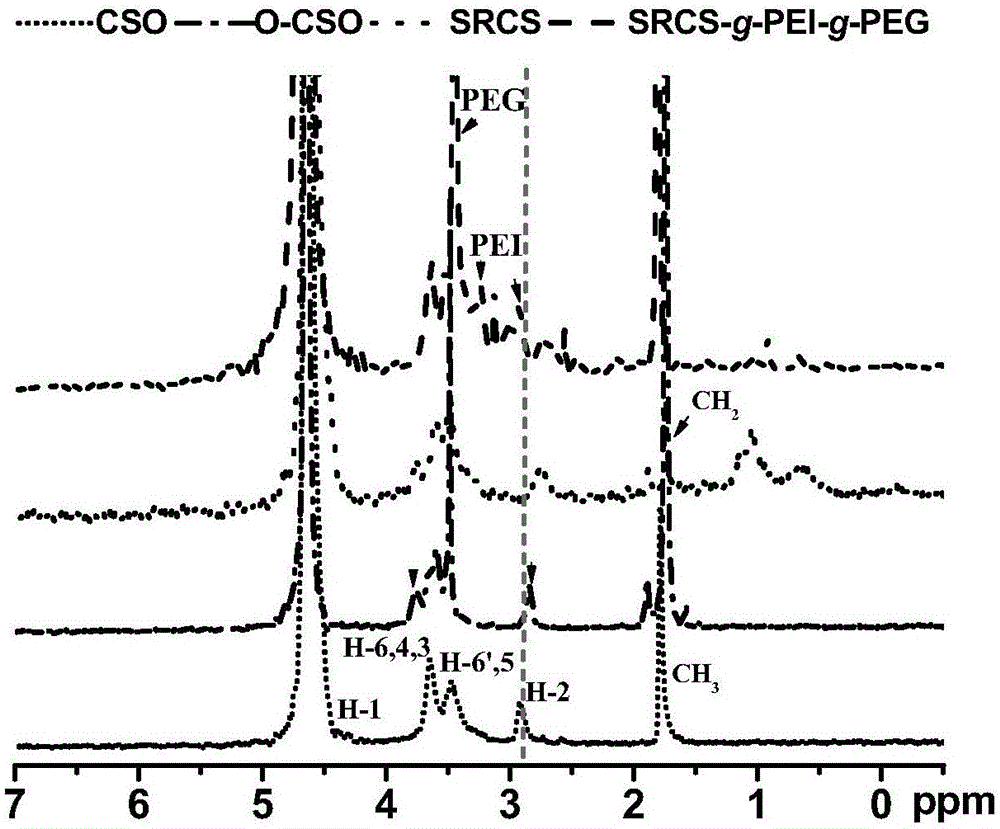
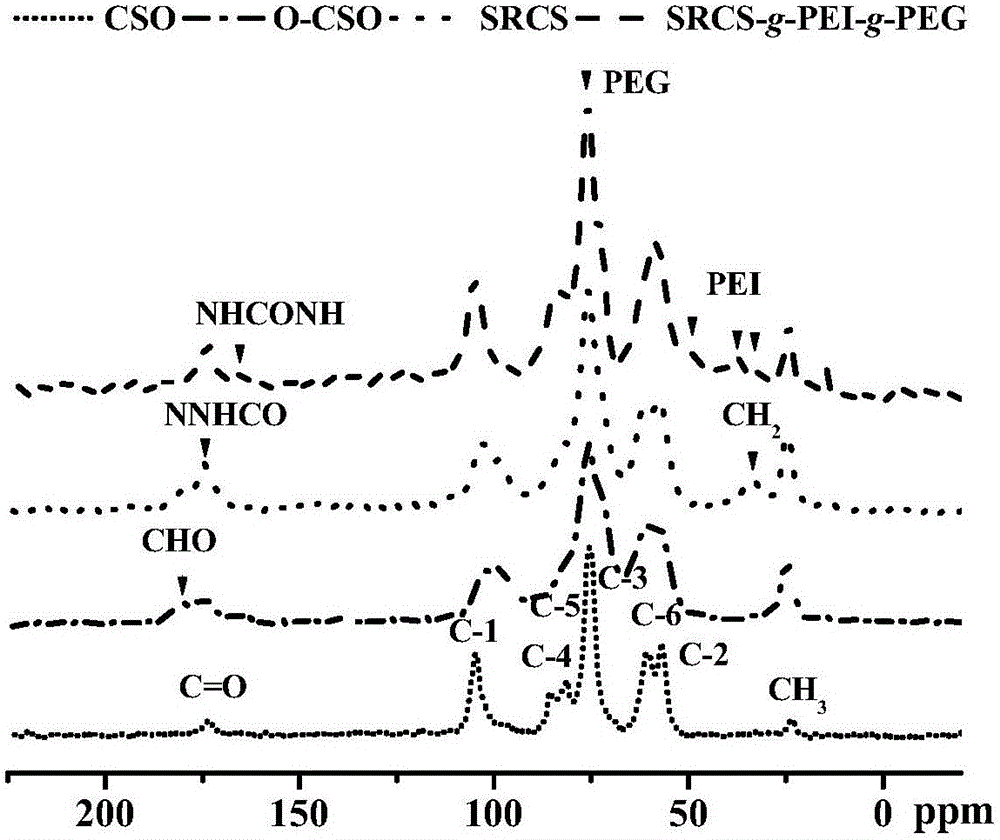
Image
Examples
Embodiment 1
[0040] Low-molecular-weight chitosan (CSO) 0.8554 g was dissolved in 50 mL of NaAc / HAc (pH=4.5) buffer solution, and ultrasonicated for 30 min to dissolve it. Dissolve 0.1093 g of sodium periodate in 50 mL of NaAc / HAc (pH=4.5) buffer solution, and sonicate for 30 min. The above two solutions were placed in an ice bath, and degassed with high-purity nitrogen for 30 minutes. The two solutions were mixed, stirred and reacted at 0-4°C for 26 hours, and 10 mL of ethylene glycol was added to stop the reaction. The reaction solution was transferred to a dialysis bag (MWCO=3000Da), dialyzed in NaAc / HAc buffer solution for 24 hours, then dialyzed in deionized water for 48 hours, and freeze-dried to obtain aldehyde-based chitosan. Weigh 0.16g of aldehyde-based chitosan, 0.10g of 3,3'-dithiobis(hydrazine propionate), three drops of glacial acetic acid, 30mL of absolute ethanol, under nitrogen protection, stir and reflux at 68°C for 42h, vacuum rotary evaporation The solvent was removed,...
Embodiment 2
[0043] Add the prepared SRCS into [BMIM]Cl ionic liquid, 80 °C oil bath N 2 Fully stir and dissolve under air protection, add CDI in an equimolar amount to the chitosan glucosamine unit, N 2 Stir and react at 80°C for 2 h under air protection, then add 0.3 mol of mPEG-NH with chitosan glucosamine unit 2 , N 2 Under air protection, react at 80°C for 2 hours, and finally add 0.45mol PEI of chitosan glucosamine unit, N 2 Stir the reaction at 80°C for 2h under gas protection. Add an appropriate amount of deionized water to the reaction solution, transfer the reaction solution mixture to a dialysis bag (MWCO=8000-14000Da), dialyze in deionized water for 3 days, and freeze-dry to obtain SRCS-g-PEI-g-PEG polymer.
Embodiment 3
[0045] Add the prepared SRCS into DMF, 40°C N 2 Stir fully under air protection to disperse evenly, add CDI in an equimolar amount to the chitosan glucosamine unit, N 2 Stir and react at 40°C for 12 hours under air protection, then add 0.3mol chitosan-glucosamine unit mPEG-NH 2 , N 2 Under air protection, react at 40°C for 12h, and finally add 0.45mol PEI of chitosan glucosamine unit, N 2 The reaction was stirred at 40°C for 12h under the protection of air. Add an appropriate amount of deionized water to the reaction solution, transfer the reaction solution mixture to a dialysis bag (MWCO=8000-14000Da), dialyze in deionized water for 3 days, and freeze-dry to obtain SRCS-g-PEI-g-PEG polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com