Method for heterologous biosynthesis of tonquinol, caffeol and ferulenol
A technology of coumaryl alcohol and coffee alcohol, applied in the biological field, can solve the problems of high cost, harsh reaction conditions, and large environmental pollution of chemical synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0034] In vitro addition of coumaric, caffeic and ferulic acids to produce coumaryl, cafestol and ferulic alcohol
[0035] Aromatic compounds are naturally synthesized by many bacteria. The pathway for the synthesis of aromatic amino acids is called the shikimate pathway.
[0036] The first step in the shikimate pathway is the condensation of PEP and E4P to produce DHAP and phosphoric acid. The synthesis of DHAP is catalyzed by DHAP synthase. The second step reaction in the shikimate pathway is the elimination of the DHAP phosphate group, yielding 3-dehydroquinic acid (DHQ). This reaction is catalyzed by DHQ synthase. The third step reaction of the shikimate pathway is the dehydration of DHQ to form 3-dehydroshikimic acid (3-DHS), which is catalyzed by DHQ dehydratase. The fourth step reaction in the shikimate pathway is the reduction of 3-DHS to form shikimate. In E. coli, this reaction is catalyzed by the NADP-dependent shikimate dehydrogenase. In the fifth step reacti...
specific Embodiment 2
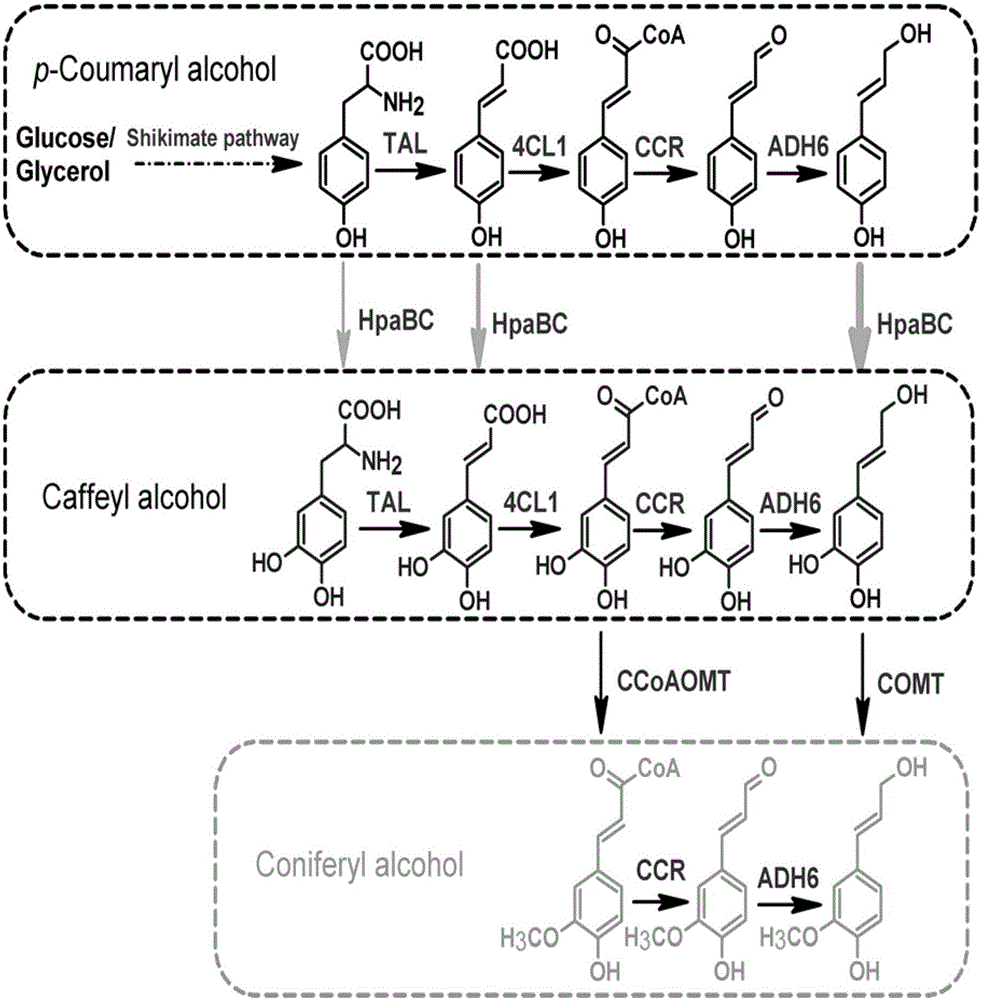
[0042] Construction of Metabolic Pathway for Microbial Heterologous Synthesis of Coumaryl Alcohol
[0043] As an important precursor of lignin synthesis, coumaryl alcohol, tyrosine synthesized by shikimate pathway, is screened from bacteria, fungi or protein engineered tyrosine ammonia lyase (TAL), coumaroyl coenzyme A ligase (4CL), cinnamoyl-CoA reductase (CCR), and alcohol dehydrogenase (ADH) catalyze the formation of coumaryl alcohol. Use PCR to obtain the corresponding gene fragments, then digest the fragments and vectors with endonucleases, perform gel recovery or column recovery on the digested fragments, and then insert the target gene into the plasmid pCS27 (middle copy) to obtain pCS - TAL-TPTA (Table 1).
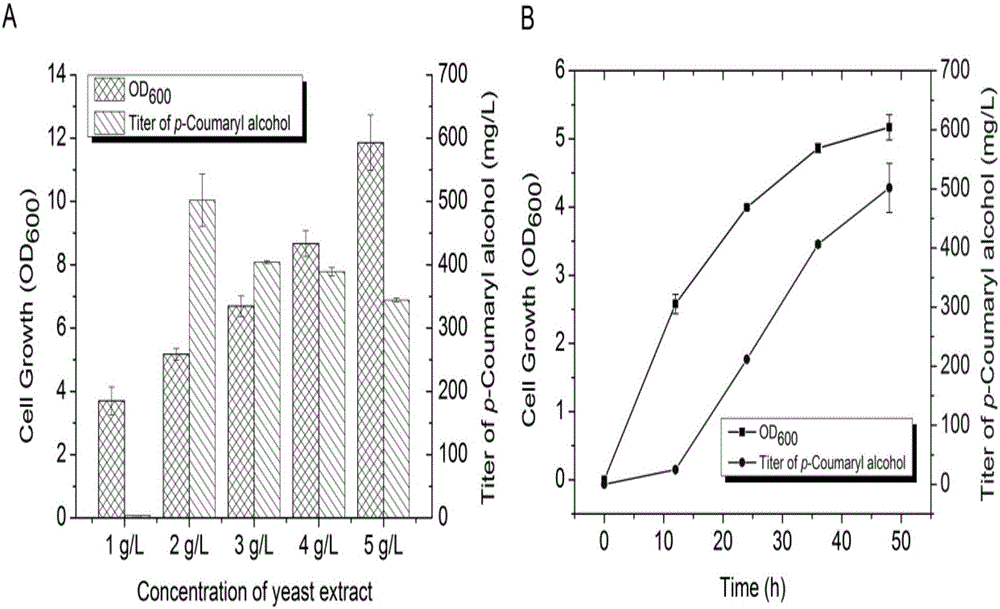
[0044] Take 1 μL of the constructed high-copy plasmid pZE-CA4 and 2 μL of the medium-copy plasmid pCS-TAL-TPTA by electrotransfection into Escherichia coli, then take 200 μL of the bacterial solution and spread it on the antibiotic plate containing Amp and Kan, an...
specific Embodiment 3
[0048] Construction of metabolic pathway for heterologous synthesis of cafestol by microorganisms
[0049] Obtain hpaBC by PCR, then digest the fragment and vector with endonuclease, recover the digested fragment from gel or column, and then insert the target gene into the plasmid pCS27 (medium copy) to obtain the pCS-HpaBC recombinant plasmid (Table 1).
[0050] Introduction of heterologous metabolic pathways into caffeol-producing microorganisms
[0051] Take 1 μL of the constructed high-copy plasmid pZE-luc and 2 μL of the medium-copy plasmid pCS-HpaBC by electrotransfection into Escherichia coli, and then take 200 μL of the bacterial solution and spread it on a plate containing the corresponding antibiotic, and incubate overnight at 37°C.
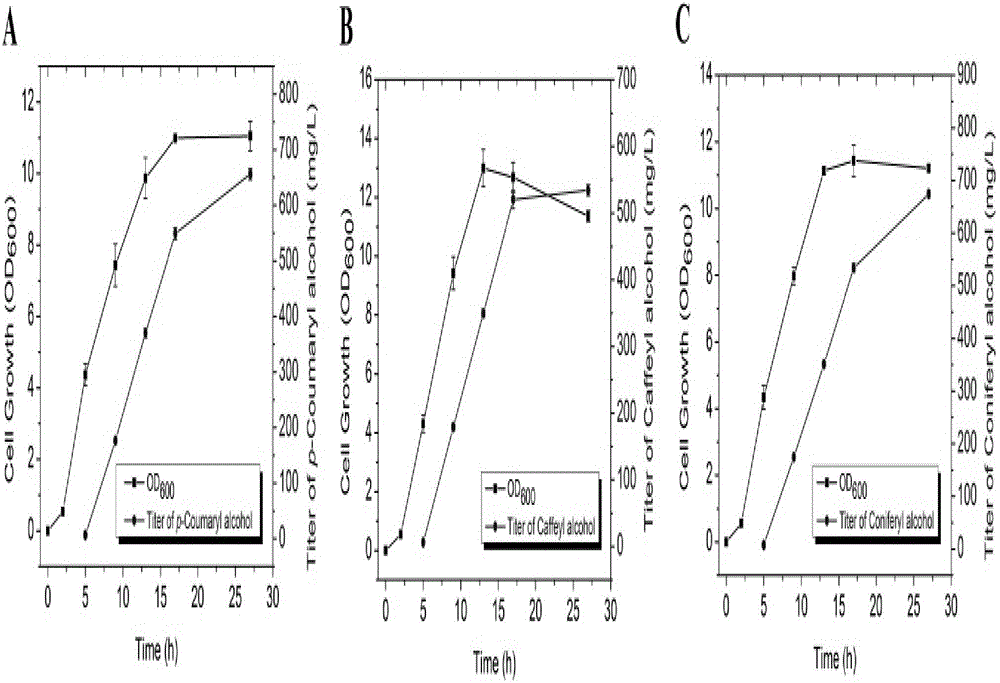
[0052] Fermentation of coffee alcohol-producing microorganisms
[0053]Pick a single colony from the plate in the genetic engineering of Escherichia coli, transfer it to 3 mL of liquid LB with corresponding resistance, and cultivate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com