Method for synthesizing natural anisaldehyde by catalyzing hydrogen peroxide to oxidize anethole
A technology of hydrogen peroxide and anisaldehyde, which is applied in the field of catalyzing the oxidation of anethole with hydrogen peroxide to synthesize natural anisaldehyde, can solve the problems of increasing process complexity and safety, complex catalyst synthesis process, and small anethole processing capacity, etc. Achieve the effect of short time, low heating temperature and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
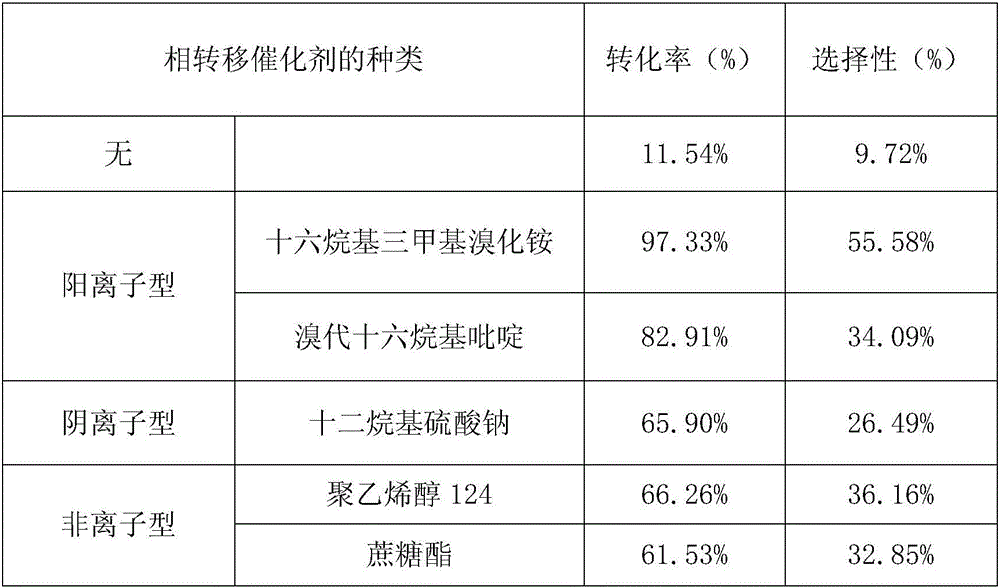
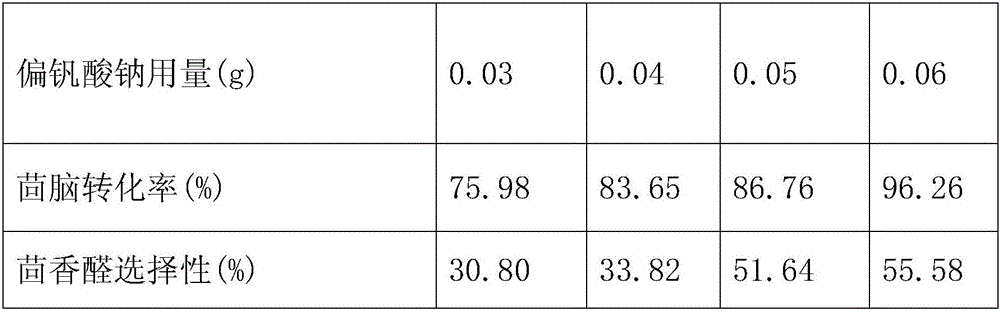
[0057] A method for catalyzing the oxidation of anethole by hydrogen peroxide to synthesize natural anisaldehyde is to use sodium metavanadate as a catalyst and hexadecyltrimethylammonium bromide as a phase-transfer catalyst in the absence of a solvent and at a certain temperature. Method for synthesizing natural anisaldehyde by oxidizing anethole with hydrogen peroxide. Specific steps are as follows:
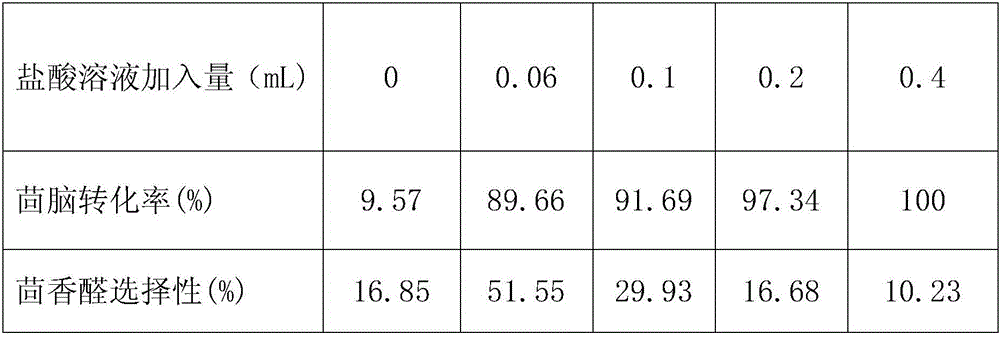
[0058] 1) Add 5.00mL of anethole, 0.0500g of sodium metavanadate, and 0.0500g of cetyltrimethylammonium bromide into a round bottom flask, and add 9.00mL of 30% hydrogen peroxide solution by mass , adjust the pH of the reaction solution to 0.60 with a hydrochloric acid solution with a concentration of 6 mol / L.
[0059] 2) Place the above-mentioned round-bottomed flask in a magnetic stirrer in a constant temperature water bath at 35°C, and heat the reaction in the water bath. After reacting for 3 hours, take 1.00 mL of the reaction solution and extract it with 10.00 mL of ethyl...
Embodiment 2
[0062] A method for catalyzing the oxidation of anethole by hydrogen peroxide to synthesize natural anisaldehyde is to use sodium metavanadate as a catalyst and hexadecyltrimethylammonium bromide as a phase-transfer catalyst in the absence of a solvent and at a certain temperature. Method for synthesizing natural anisaldehyde by oxidizing anethole with hydrogen peroxide. Specific steps are as follows:
[0063] 1) Add 5mL of anethole, 0.0500g of sodium metavanadate and 0.0500g of cetyltrimethylammonium bromide into a round bottom flask, and add 9.00mL of 30% hydrogen peroxide solution by mass percentage, The pH of the reaction solution was adjusted to 0.60 with a hydrochloric acid solution with a concentration of 6 mol / L.
[0064] 2) Place the above-mentioned round-bottomed flask in a magnetic stirrer in a constant temperature water bath at 25°C, and heat the reaction in the water bath. After reacting for 3 hours, take 1.00 mL of the reaction solution and extract it with 10.00...
Embodiment 3
[0067] A method for catalyzing the oxidation of anethole by hydrogen peroxide to synthesize natural anisaldehyde is to use sodium metavanadate as a catalyst and hexadecyltrimethylammonium bromide as a phase-transfer catalyst in the absence of a solvent and at a certain temperature. Method for synthesizing natural anisaldehyde by oxidizing anethole with hydrogen peroxide. Specific steps are as follows:
[0068] 1) Add 5.00mL of anethole, 0.0500g of sodium metavanadate, and 0.0500g of cetyltrimethylammonium bromide into a round bottom flask, and add 9.00mL of 30% hydrogen peroxide solution by mass , adjust the pH of the reaction solution to 0.60 with a hydrochloric acid solution with a concentration of 6 mol / L.
[0069] 2) Place the above-mentioned round-bottomed flask in a magnetic stirrer in a constant temperature water bath at 40°C, and heat the reaction in the water bath. After reacting for 3 hours, take 1.00 mL of the reaction solution and extract it with 10.00 mL of ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com