A kind of Fe2+ anodic oxidation and cathodic reduction parallel production method of H2
A technology of anodic oxidation and process method, which is applied in the direction of chemical instruments and methods, photographic process, photographic auxiliary process, etc., and can solve the problems of difficult regeneration, high production cost, system pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
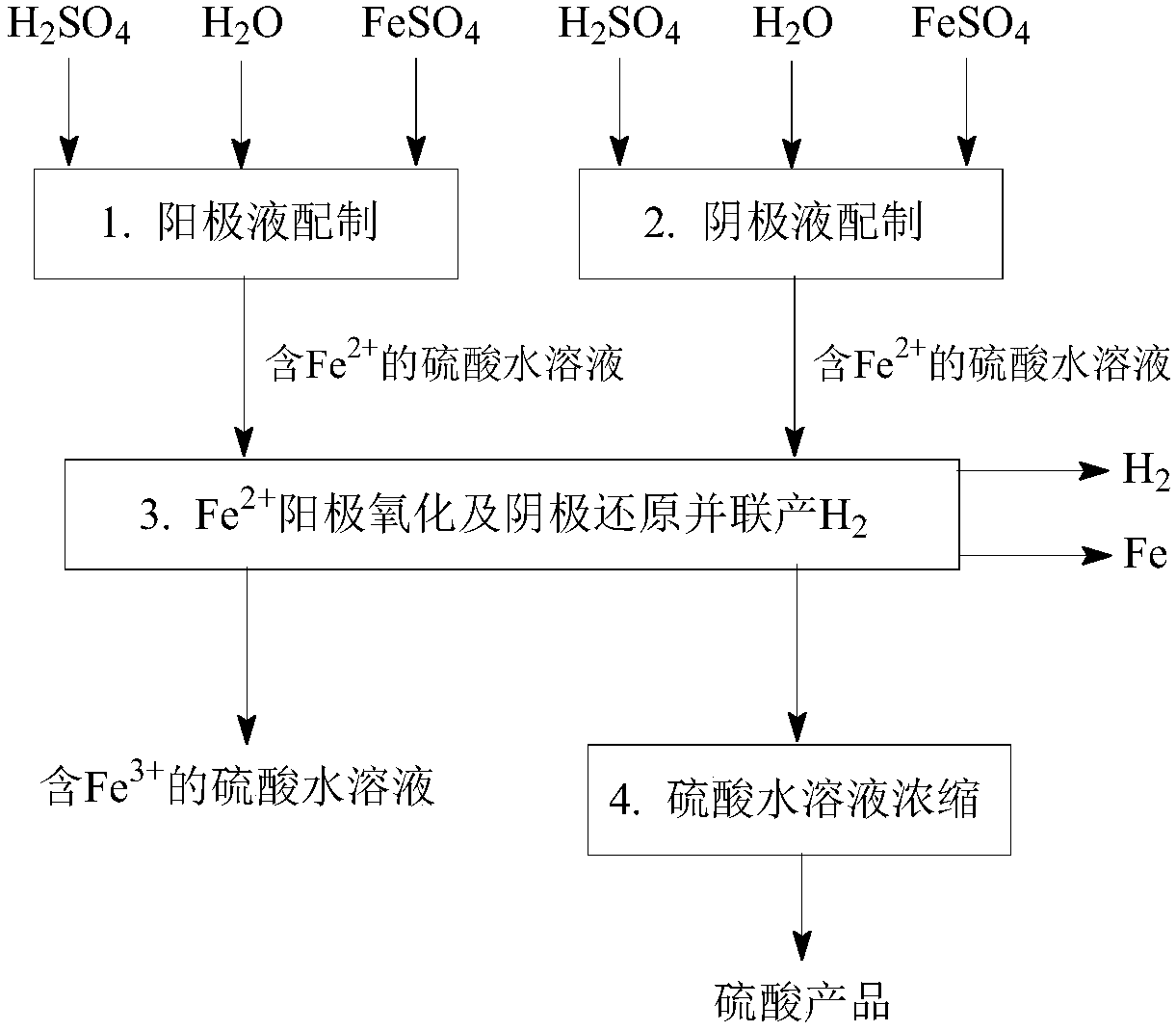
[0092] like figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0093] (1) Preparation of anolyte: mix sulfuric acid with water to obtain an aqueous solution of sulfuric acid, then dissolve ferrous sulfate in the aqueous solution of sulfuric acid to obtain a solution containing 0.2mol / L H 2 SO 4 and 0.2mol / L Fe 2+ The anolyte, the operating temperature is 20 ℃.
[0094] (2) catholyte preparation: will contain Fe 3+ The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then the ferrous sulfate is dissolved in the sulfuric acid aqueous solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and 0.02mol / L Fe 3+ The catholyte, the operating temperature is 20 ℃.
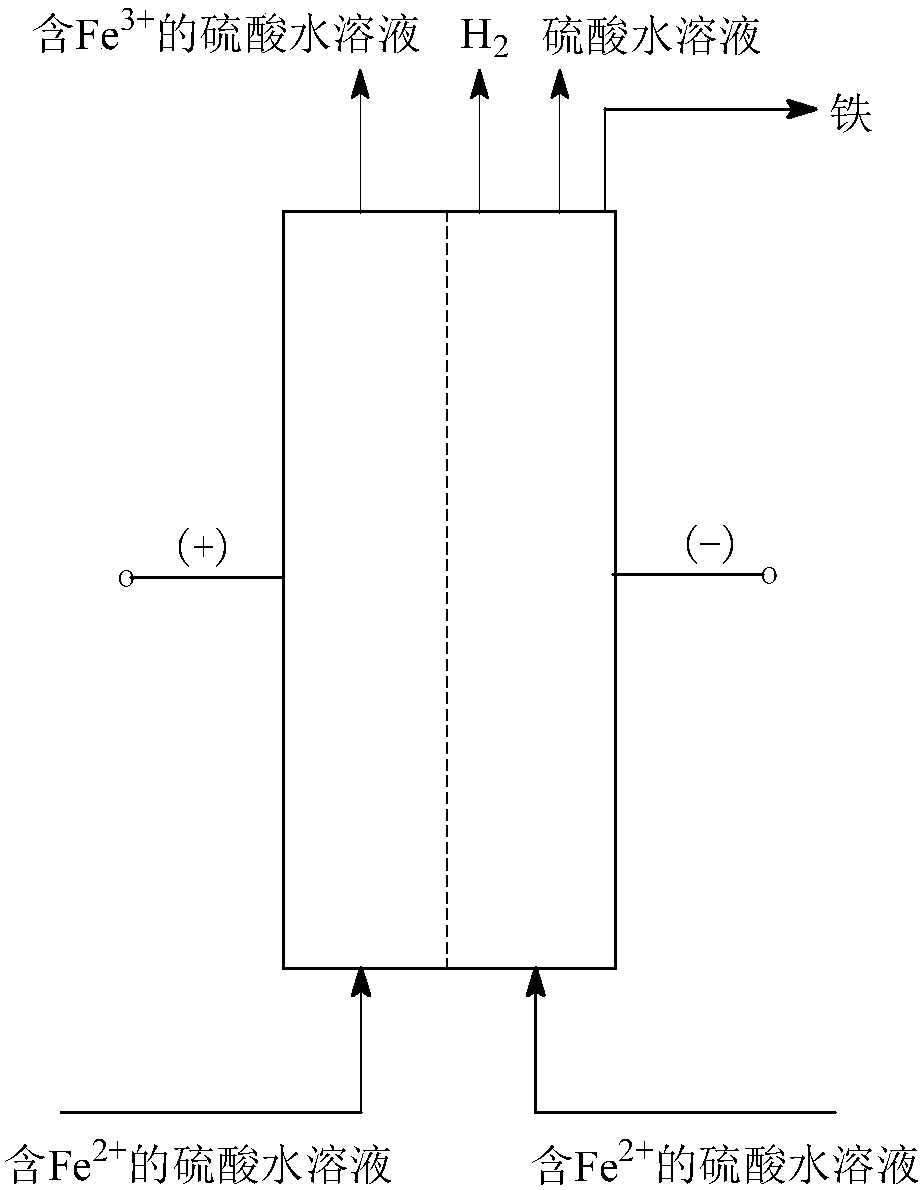
[0095] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In an electrochemical reactor with a sulfonic acid type cation exchange membrane as the isolation membrane, sulfu...
Embodiment 2
[0097] like figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0098] (1) Anolyte preparation: will contain Fe3+ The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then ferrous sulfate is dissolved in the solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and Fe 3+ For anolyte with concentration ≤0.2mol / L, the operating temperature is 60°C.
[0099] (2) catholyte preparation: will contain Fe 3+ The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then ferrous sulfate is dissolved in the solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and 0.02mol / L Fe 3+ The catholyte operating temperature is 60°C;
[0100] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In an electrochemical reactor with a sulfonic acid type cation exchange membrane as a separator, sulfuric acid as a supporting electr...
Embodiment 3
[0102] like figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0103] (1) Anolyte preparation: will contain Fe 2+ and Fe 3+ The sulfuric acid aqueous solution is directly used as the anolyte, and the solution composition is 6.0mol / L H 2 SO 4 , 0.8mol / L Fe 2+ and 0.1mol / L Fe 3+ , operating temperature is 40°C.
[0104] (2) catholyte preparation: will contain Fe 2+ and Fe 3+ The sulfuric acid aqueous solution is directly used as the catholyte, and the solution composition is 8.0mol / L H 2 SO 4 , 1.0mol / L Fe 2+ and 0.02mol / L Fe 3+ , operating temperature is 40°C.
[0105] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In a three-dimensional fixed-bed electrode electrochemical reactor with a sulfonic acid-type cation-exchange membrane as a separator, sulfuric acid as a supporting electrolyte, and a graphite electrode as an anode, the operating c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com