PH-sensitive amphipathic graft copolymer POEAd-g-MPEG, preparation method and application of graft copolymer
A graft copolymer, poead-g-nh2 technology, applied in the field of synthesis of new pH-sensitive amphiphilic graft copolymers, can solve the problems of weak acid sensitivity, low stability, and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Polyorthoester Copolymer POEAd-g-COF 3 The synthetic method of is realized through the following steps:
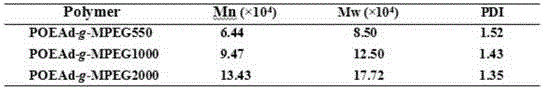
[0030] Under a nitrogen atmosphere, add 1.01g (3.28MmoL) 4,4'-dimethethyleneoxy-bis-(2-aminoethoxy-1,3-dioxolane) to a 50ml eggplant-shaped bottle, 1.90 g (3.28Mmol) 2-N-trifluoroacetyl-1,3-di-(4'-succinimidyl succinate) propylene glycol ester, 1.5mL triethylamine, 10mL anhydrous N,N-di Methylformamide was reacted at 30°C for 7 days, a small amount of dichloromethane was added, and anhydrous ether was settled twice to obtain 1.87 g of a white solid product with a yield of 86.6%. 1 H NMR (400MHz, CDCl 3 ,δ,ppm):2.55-2.61(m,8H,OOC-CH2-CH2-COO),3.41(s,4H,H2N-CH2),3.55-3.78(m,8H,N-CH2-CH2-O, CH-CH2-O),4.02-4.13(m,8H,O-CH2-CH,NH-CH-CH2-O),4.31-4.47(m,4H,O-CH2-CH2-CH-O),5.82 -5.83(d,2H,CH-(O)3),6.47(s,3H,OC-NH)( figure 1 ).
Embodiment 2
[0032] Polyorthoester copolymer POEAd-g-NH with side chain amino group 2 , the synthetic method is realized through the following steps:
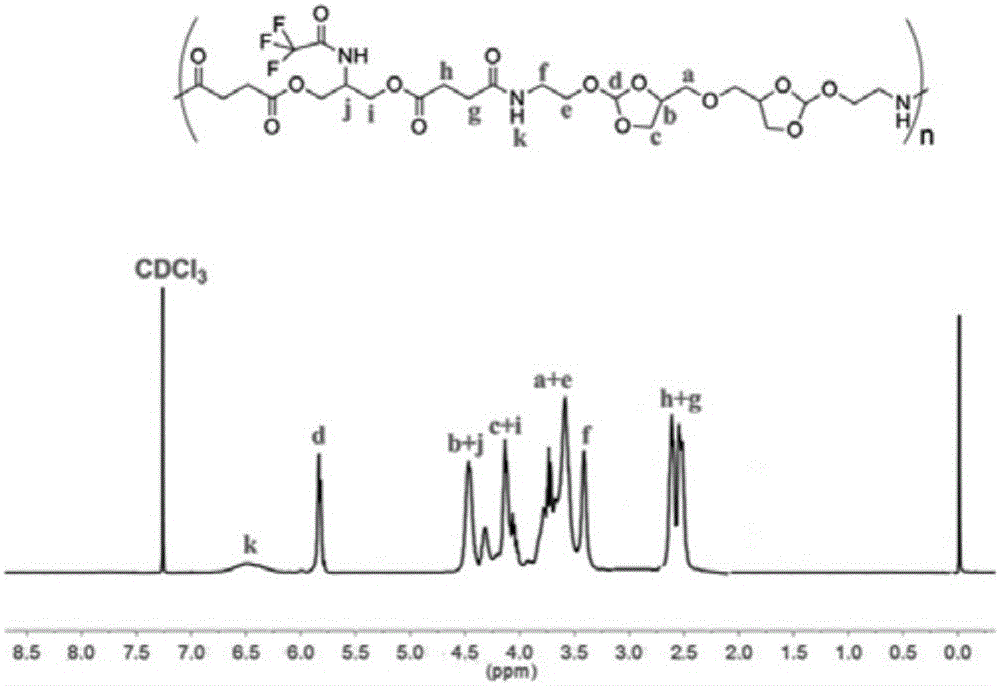
[0033] 1.87g main chain polyorthoester copolymer POEAd-g-COF 3 Dissolve with 50mL tetrahydrofuran, add to 300mL 7% sodium carbonate aqueous solution, magnetically stir and react for 6 hours, remove tetrahydrofuran by distillation under reduced pressure, and then use a 3500Da dialysis belt to dialyze in a small amount of triethylamine aqueous solution for 48 hours (change every six hours) dialysate), and finally freeze-dried to obtain the target polymer POEAd-g-NH 2 1.52 g, yield 81.3%. 1 HNMR (400MHz, DMSO-d 6 ):δ(ppm)2.27-2.38(m,8H,OOC-CH 2 -CH 2 -COO),3.15-3.20(m,4H,H 2 N-CH 2 ),3.41-3.66(m,8H,N-CH 2 -CH 2 -O,CH-CH 2 -O),4.02-4.06(t,8H,O-CH 2-CH,NH-CH-CH 2 -O),4.20-4.40(m,4H,O-CH 2 -CH 2 -CH-O),5.83-5.85(d,2H,CH-(O) 3 )( figure 2 ).
Embodiment 3
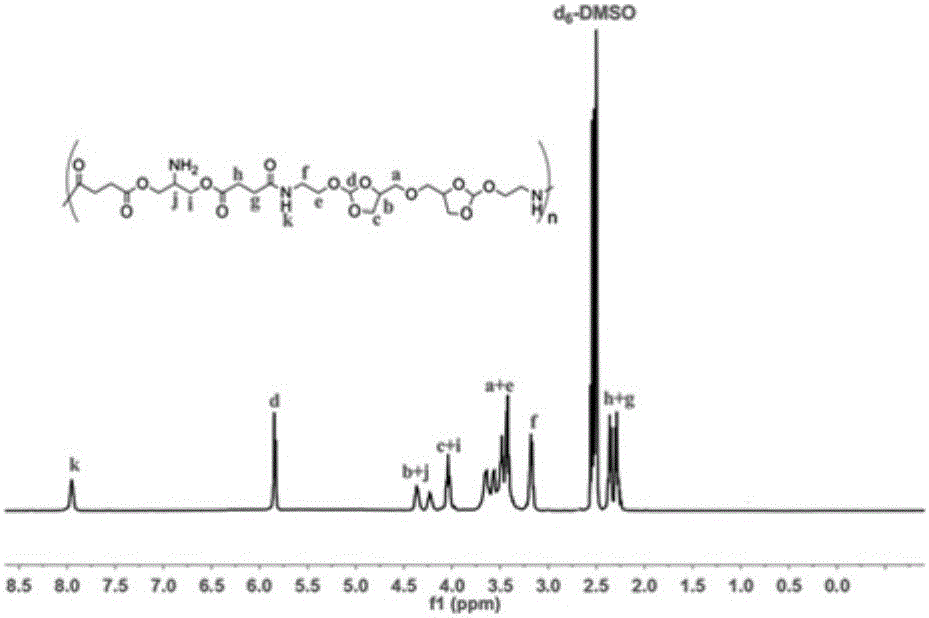
[0035] The synthetic method of pH sensitive amphiphilic graft copolymer POEAd-g-MPEG550, realizes by following steps: the copolymer POEAd-g-NH 2 0.56g, polyethylene glycol monomethyl ether active ester (molecular weight: 550) 2.73g, 4.57Mmol, 1mL triethylamine, accurately weighed and put into a dry eggplant-shaped reaction bottle, and then the reaction bottle was placed in a 30°C React in an oil bath. After 24 hours of reaction, use a 3500Da dialysis belt to dialyze in an aqueous solution with a small amount of triethylamine for 48 hours (change the dialysate every six hours), and finally freeze-dry to obtain the target polymer. 1 HNMR (400MHz, DMSO-d 6 ):δ(ppm)2.29-2.43(m,8H,OOC-CH 2 -CH 2 -COO),3.06-3.10(m,4H,H 2 N-CH 2 ),3.18-3.21(m,8H,N-CH 2 -CH 2 -O,CH-CH 2 -O),3.24(s,3H,PEG-OCH 3 ), 3.51(s, MPEG), 4.02-4.06(t, 8H, O-CH 2 -CH,NH-CH-CH 2 -O),4.20-4.40(m,4H,O-CH 2 -CH 2 -CH-O),5.83-5.85(d,2H,CH-(O) 3 )( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com