Phenothiazine derivative, preparation method and application thereof in dye-sensitized solar cells
A solar cell and phenothiazine technology, applied in the field of phenothiazine derivatives, can solve the problem that solar energy cannot meet the energy demand, etc., and achieve the effects of high photoelectric conversion efficiency, excellent performance and large light absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
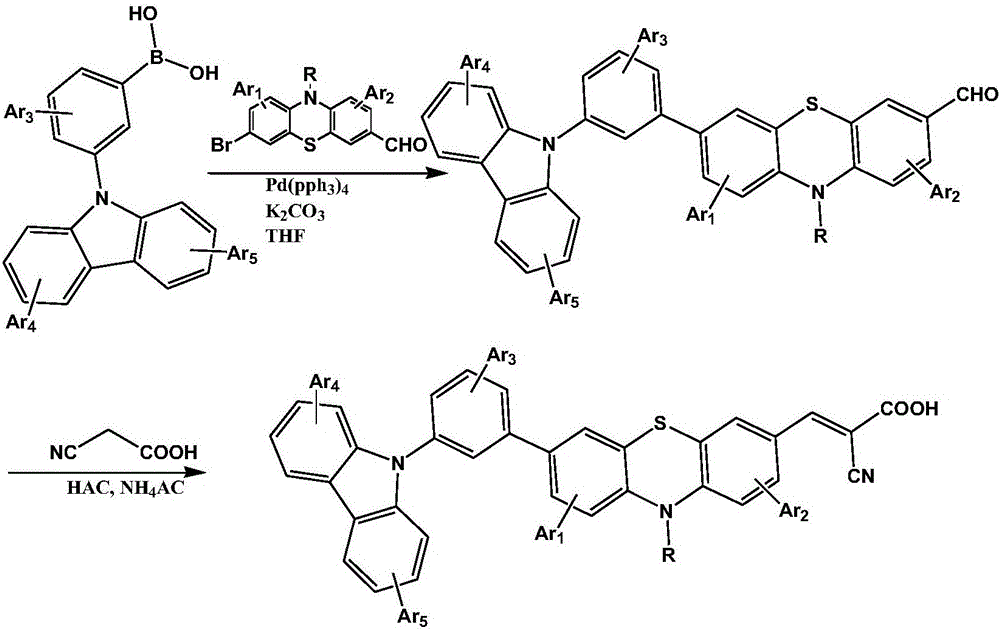
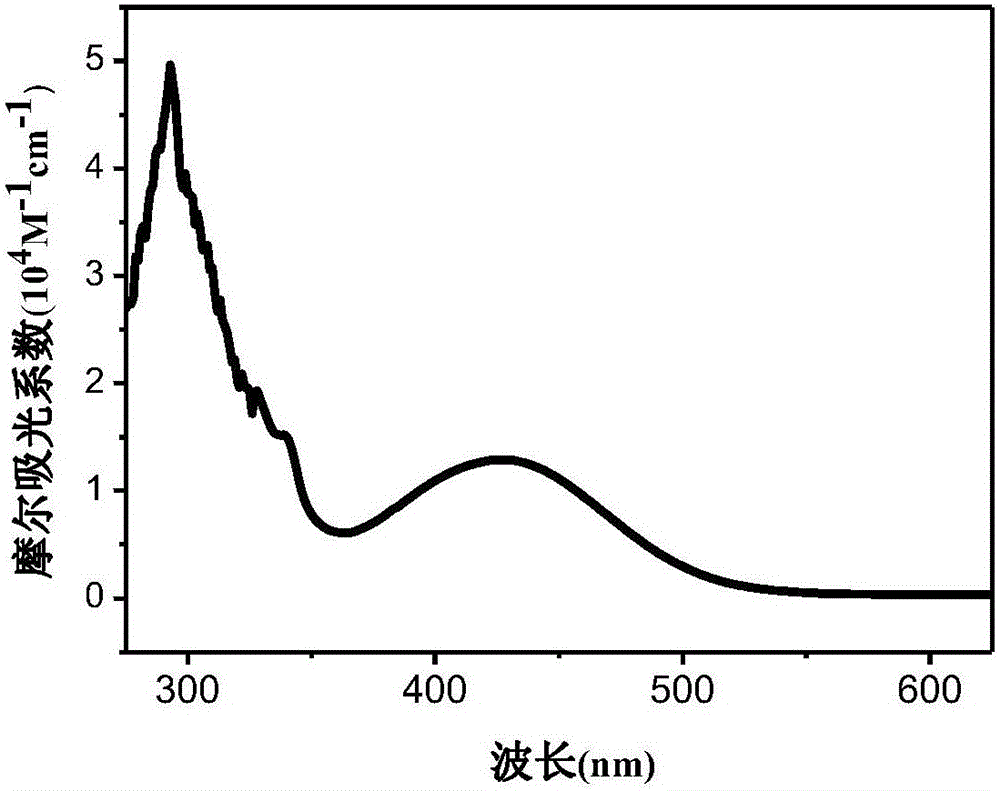
[0048] The phenothiazine pure organic fuel in this example is 3-(9H-carbazol-9-yl)phenyl-7-(N-ethyl-10H-phenothiazine)-3-cyanoacetic acid (abbreviated as CBPTZ2), its molecular formula is:
[0049]
[0050] The preparation method of CBPTZ2 comprises the following three steps:
[0051] The first step: the synthesis of phenothiazine π bridge, and its specific process is:
[0052] The solid phenothiazine, bromoethane and strong base were dissolved in DMSO in a molar ratio of 1:1.5:0.6, reacted at room temperature for 8 hours, quenched with water and extracted with dichloromethane to give N -Ethyl phenothiazine, dissolve N-ethyl phenothiazine in N,N-dimethylformamide, and simultaneously add the treated phosphorous oxychloride and N,N-dimethylformamide solution dropwise, The treatment method is as follows: mixing phosphorus oxychloride and N,N-dimethylformamide at a ratio of 1:3-4 to reddish brown, and reacting at 80° C. for 20 hours to obtain N-ethylphene substituted with 3-p...
Embodiment 2
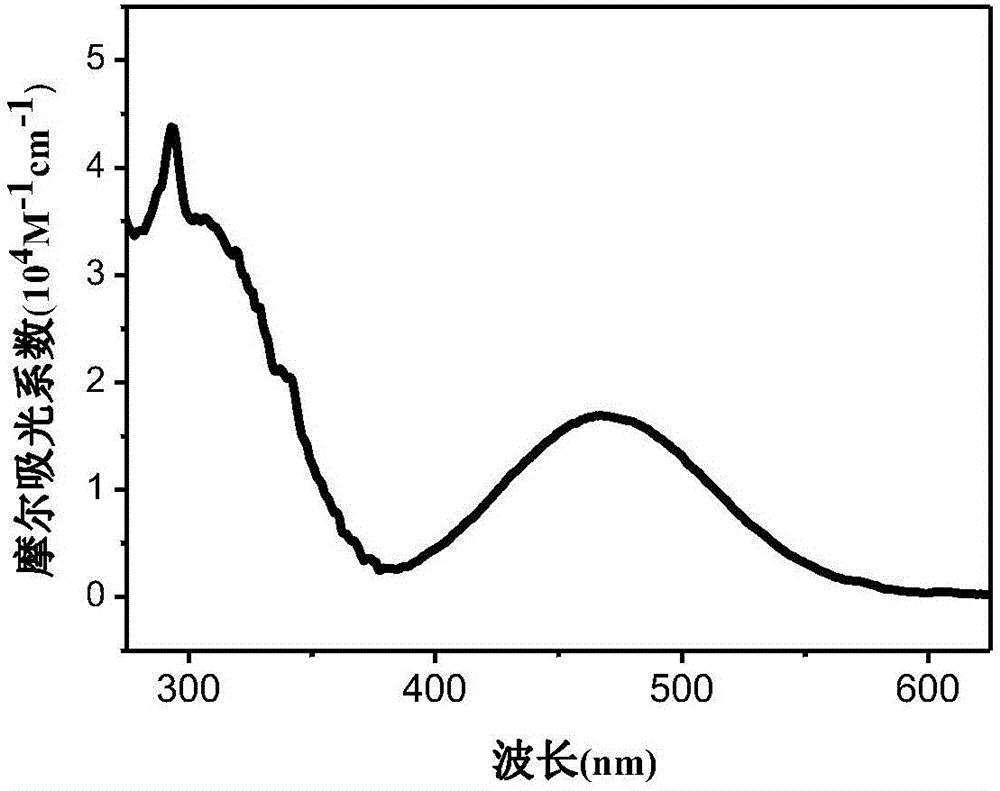
[0059] The phenothiazine pure organic fuel in this example is 3-(9H-carbazol-9-yl)phenyl-7-(N-hexyl-10H-phenothiazine)-3-cyanoacetic acid (CBPTZ6 for short) ), its molecular formula is:
[0060]
[0061] The preparation method of CBPTZ6 comprises the following three steps:
[0062] The first step: the synthesis of phenothiazine π bridge, and its specific process is:
[0063] The phenothiazine, bromohexane and the strong base solid were dissolved in DMSO in a molar ratio of 1:1.5:0.6, reacted at room temperature for 8 hours, quenched with water and extracted with dichloromethane to give N -hexylphenothiazine, N-hexylphenothiazine is dissolved in N,N-dimethylformamide, while the treated phosphorous oxychloride and N,N-dimethylformamide solutions are added dropwise, said The treatment method is to mix phosphorus oxychloride and N,N-dimethylformamide at a ratio of 1:3-4 to reddish brown, and react at 80 °C for 20 hours to obtain N-hexylphenothiazine derivatives substituted by...
Embodiment 3
[0070] The phenothiazine pure organic fuel in this example is also CBPTZ2.
[0071] The preparation method of CBPTZ2 comprises the following three steps:
[0072] The first step: the synthesis of phenothiazine π bridge, and its specific process is:
[0073] The phenothiazine, bromoethane and the strong base solid were dissolved in DMSO in a molar ratio of 1:0.5:0.5, reacted at room temperature for 6 hours, quenched with water and extracted with dichloromethane to give N -Ethyl phenothiazine, dissolve N-ethyl phenothiazine in N,N-dimethylformamide, and simultaneously add the treated phosphorous oxychloride and N,N-dimethylformamide solution dropwise, The treatment method is to mix and stir phosphorus oxychloride and N,N-dimethylformamide at a ratio of 1:3-4 to reddish brown, and react at 90° C. for 15 hours to obtain N-ethylphene substituted by the 3-position aldehyde group. Thiazine derivative; dissolve N-ethylphenothiazine-3-aldehyde in chloroform, add bromine reagent NBS i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com