Magnetotactic immune cells and construction method and application thereof
An immune cell and magnetic technology, applied in the field of magnetotactic immune cells and their construction, can solve the problems of inability to enhance the penetration and retention of immune cells, restrict the enrichment efficiency of immune cells, and time-consuming and labor-intensive operation procedures, and achieve low toxicity and magnetic The effect of high responsiveness and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment one: Fe 3 o 4 Preparation of nanoparticles
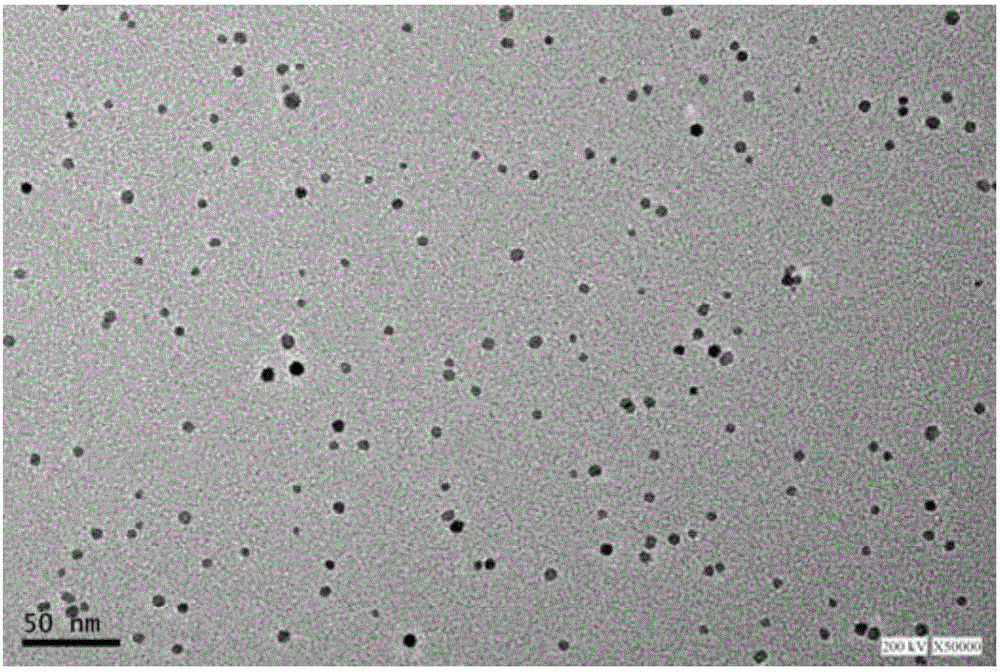
[0039] Preparation of Fe by Polyol Method 3 o 4 Nanoparticles: Put 720mg of iron acetylacetonate in a 100mL three-neck bottle with a condenser, vacuumize and nitrogen gas repeatedly three times, then inject 40mL of triethylene glycol into the reaction system, stir magnetically, heat in a sand bath, and slowly raise the temperature to 180°C, keep warm for 30min; then rapidly raise the temperature to 278°C (the boiling point of triethylene glycol), and reflux for 30min to obtain a black magnetic fluid; cool to room temperature, and use a mixed solution of ethanol:ethyl acetate (volume ratio 1:10) Repeated washing three times, after magnetic separation, Fe with a particle size of 1-25nm 3 o 4 nanoparticles. Will Fe 3 o 4 Nanoparticles are re-dispersed in ethanol and stored at low temperature for future use.
Embodiment 2
[0040] Example 2: Pullulan modified Fe 3 o 4 Preparation of Magnetic Nanoparticles (MMNPs)
[0041] Take Fe 3 o 4 100mg of nanoparticles, dispersed in 10mL of n-hexane, disperse 300mg of pullulan with a molecular weight of 2 to 100,000 Daltons in 30 to 100mL containing 0.5 to 2.0wt% polyvinyl alcohol (PVA) and 0.2 to 2.0wt% In the aqueous solution of sodium dodecylbenzenesulfonate (SDBS), use a milk homogenizer to fully emulsify at a speed of 15,000 rpm for 2 minutes, then use a mechanical stirrer to stir at a speed of 600 rpm for 4 to 8 hours to volatilize n-hexane, The nanoparticles were solidified and formed, and then dialyzed with deionized water for 48 hours to obtain purified pullulan-modified Fe 3 o 4 magnetic nanoparticles.
[0042] By adjusting the Fe 3 o 4 The ratio of rice grains to polysaccharides, the concentration of emulsifiers and surfactants, the strength of emulsification, and the selection of dispersing solvents can regulate the particle size (tens o...
Embodiment 3
[0043] Example 3: Construction of magnetotactic T lymphocytes (mt-T)
[0044] 1. Isolation and expansion of human peripheral blood T lymphocytes
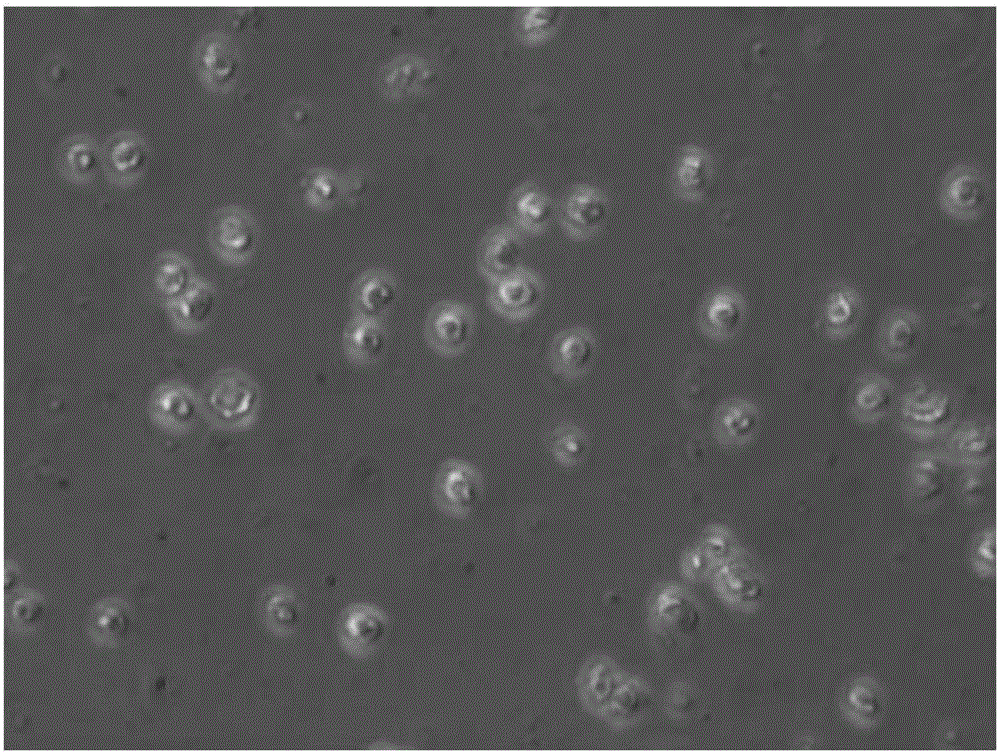
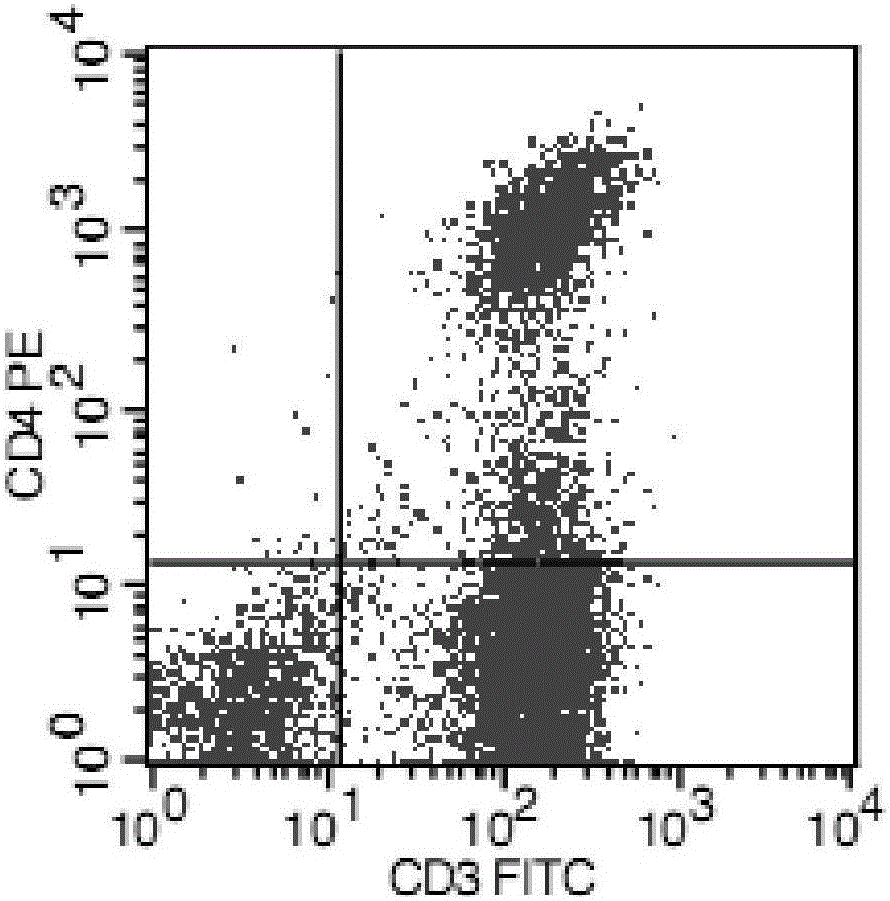
[0045] Human peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation, separated and purified by nylon wool column and sorted by immunomagnetic beads, and CD3+ T cells were isolated, and then cultured in serum-free and low-sugar DMEM medium at 37°C, 5% CO 2 conditions for 72 hours. The isolated T cells have good activity, such as figure 1 with figure 2 shown.
[0046] 2. Construction of magnetotactic T lymphocytes (mt-T)
[0047] Take the density in the exponential growth period as 5~10×10 5 5 mL of T cell suspension per mL, at 37°C, 5% CO 2 conditions for 24 hours. The MMNPs prepared in Example 2 were dispersed in serum-free and low-sugar DMEM medium, and then added to the cultured T cell suspension. The final concentration of MMNPs in the T cell suspension was 20-100 μg / mL. At 37°C, 5% C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com