Novel microbial product having antifungal activity
A technology of drugs and compounds, applied in the direction of microorganisms, microorganisms, antifungal agents, etc., can solve the problems of obtaining new drug candidate compounds, obtaining promising new drug candidate compounds, etc., and achieve low reactivity and strong antifungal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Isolation and analysis of Streptomyces sp.A84 strain
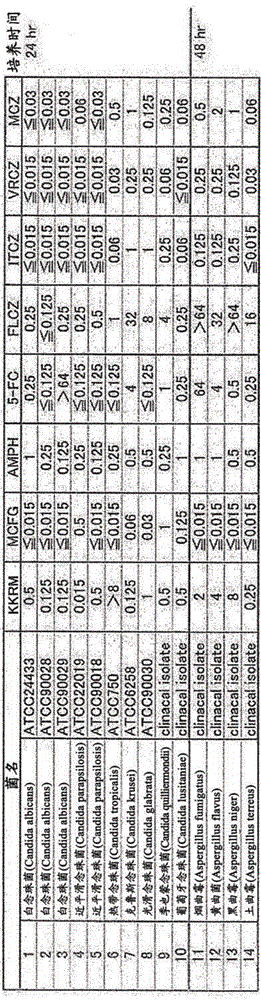
[0056] The isolated source was collected from the sea sand of Kakeroma Island, Kagoshima Prefecture. Strains were isolated from the collected samples, and the isolated microbial strains were evaluated and screened with antifungal activity as an index. As a result, promising strains were obtained, which were further examined in detail.
[0057] The strain was analyzed by phylogenetic analysis of 16S rRNA, and it was found that the producing bacteria were actinomycetes of the genus Streptomyces, and the related species were Streptomyces nodosus (98.8% homology) and Streptomyces glomeratus ) (99.0% homology). Since the nucleotide sequence homology with the closest relative Streptomyces glomeratus is 99.0%, this indicates the possibility of a new species. This strain was named as Streptomyces sp.A84 strain. The Streptomyces sp.A84 strain was deposited in Kisarazu City, Chiba Prefecture, at 2-5-8, 2-5-8,...
Embodiment 2
[0058] Example 2: Study of production medium for A84 strain
[0059] The production medium for the A84 strain was investigated.
[0060] As a result of the study of the medium, good productivity was shown in the following medium.
[0061] Medium composition:
[0062] Starch 4%
[0063] Case peptone 0.1%
[0064] NaH 2 PO 4 0.1%
[0065] Artificial seawater (MARINE ART SUPER FORMULA 1, manufactured by Tomita Pharmaceutical Co., Ltd.) 0.384%
[0066] pH 7.0
[0067] Cultivation conditions: A medium (100 ml) was filled into a 300-ml volume Erlenmeyer flask, and cultured at 28° C., 200 rpm for 2-4 days.
Embodiment 3
[0068] Example 3: Isolation, purification and analysis of active ingredients
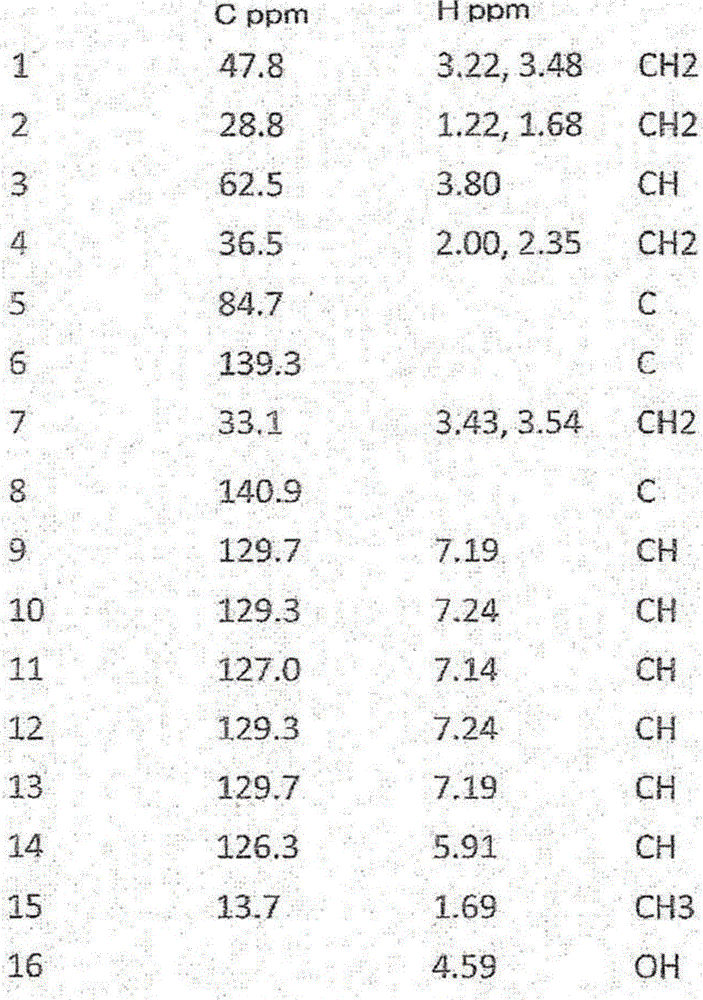
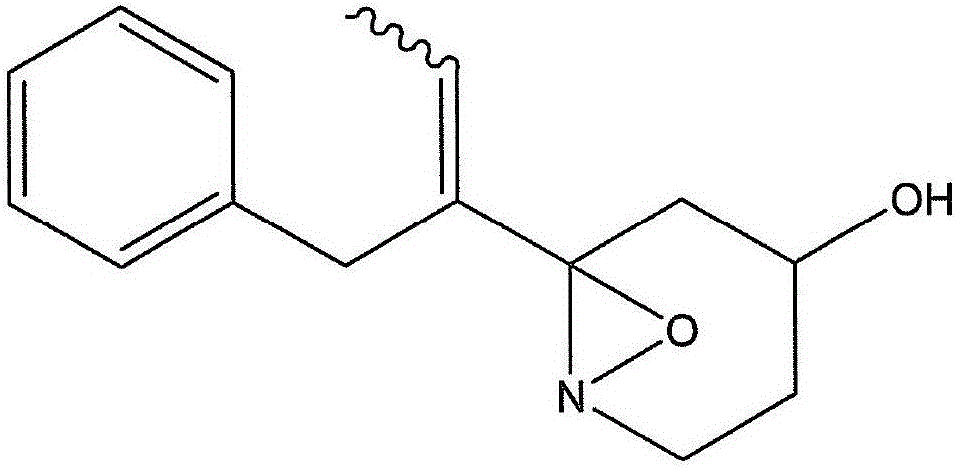
[0069]The A84 strain was inoculated into a 300 ml volumetric Erlenmeyer flask supplemented with 100 ml of the medium having the composition described in Example 2, and cultured at 28° C., 200 rpm (rotary shaker) for 3 days. The broth was solvent extracted with an equal amount of ethyl acetate, and then the ethyl acetate was concentrated. The dried sample was dissolved in a small amount of methanol, and purified by column chromatography by HPLC using an ODS column (purity 95% or more, 100 μg / 1L).
[0070] LC / MS analysis, NMR analysis and optical rotation measurements of the active ingredient were carried out. The measurement conditions are as follows:
[0071] LC / MS:
[0072] Device: Model Agilent6540
[0073] Ion source: AJS-ESI, ESI, APC1
[0074] NMR:
[0075] Device: ECA-600 (manufactured by JEOL Ltd.)
[0076] Magnet: SCM 14.o1T
[0077] Bore diameter: 54mm
[0078] Probe: 3mm, 5mm, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com