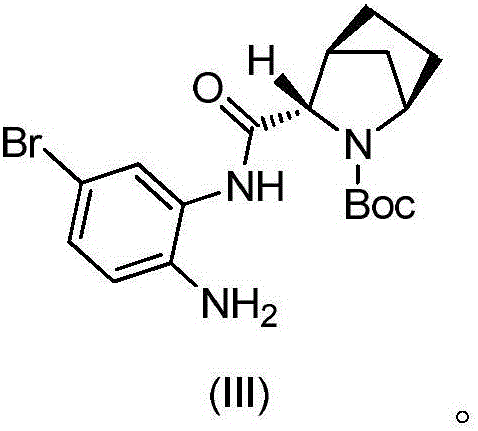
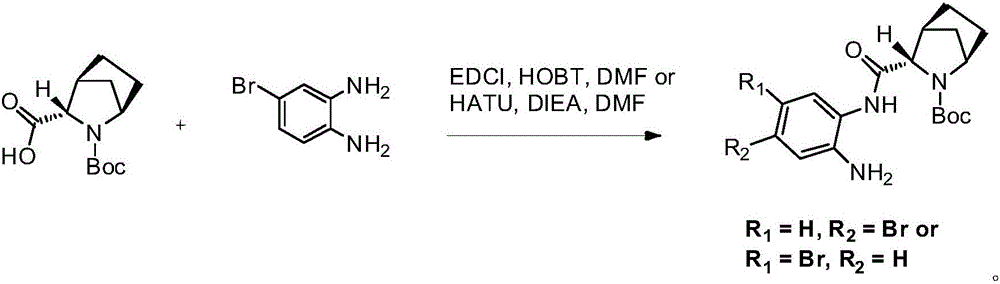
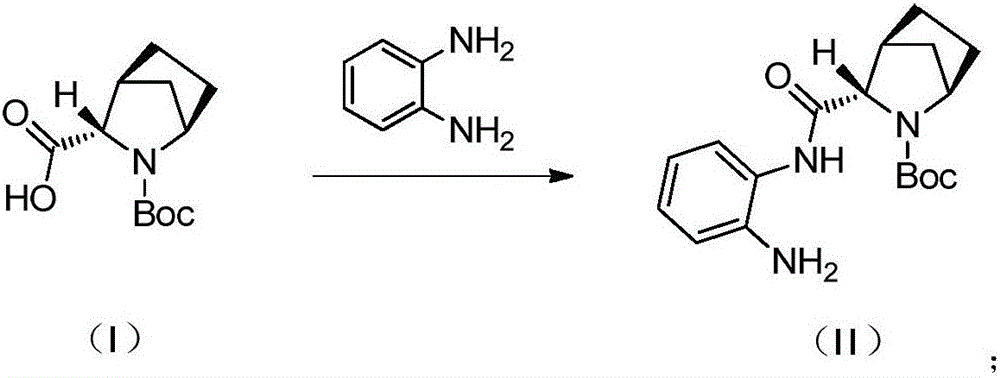
Preparation method of ledipasvir intermediate
A technology of intermediates and compounds, which is applied in the field of preparation of ledipasvir intermediates, can solve the problems of price reaction raw materials, condensation reagent reaction purification difficulties, etc., and achieve the goal of not being easy to oxidize and discolor, reducing the residue of impurities, and improving the reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] a) Add compound 1 (30.0g, 122.5mmol), MTBE (300ml) and NMM (14.8g, 146.9mmol, 1.2eq) into a 500ml three-necked flask, lower the internal temperature to T1 HNMR (400MHz, CDCl 3 ): δ8.52(s,1H),7.35(s,1H),7.01(s,1H),6.78-6.75(m,2H),4.15(s,1H),3.92-3.87(d,J=20.0 Hz,2H),3.03(s,1H),1.87-1.77(m,2H),1.70-1.68(m,2H),1.65-1.60(m,2H),1.50(s,9H),1.40-1.37( d, J=12Hz, 1H).
[0037] b) Add compound 3 (29.0g, 87.6mmol) into a 250ml three-necked flask, add DMF150ml, lower the internal temperature to T=-5-0°C, add NBS (15.6g, 87.6mmol, 1eq), and react for 0.5h after adding , the reaction was quenched with water, extracted with ethyl acetate (200ml*2), the organic layers were combined, washed with water, and concentrated under reduced pressure to obtain compound 4. Compound 4 was dissolved in MTBE (300ml), added acetic acid (26.3g, 438mmol, 5eq), heated to 55°C for 10h, washed with 5% NaOH aqueous solution, separated, washed the organic layer with water, concentrated to dryness, and ad...
Embodiment 2
[0039] a) Add compound 1 (30.0g, 122.5mmol) into a 500ml three-necked flask, add ethyl acetate (300ml), add NMM (14.8g, 146.9mmol, 1.2eq), and lower the internal temperature to T=-10°C-- 5°C, add isobutyl chloroformate (16.7g, 122.5mmol, 1eq) dropwise, react at temperature for 5h, then add compound 2, keep warm for one hour, return to room temperature and react for 8h, add water to quench, and use ethyl acetate ( 200ml*2) extraction, combined organic layers, washed the organic layer with water, and concentrated under reduced pressure to obtain the crude product of compound 3.
[0040] b) Add the crude product of compound 3 (40g, 120.8mmol) into a 500ml three-necked flask, add DMF200ml, cool the ice-salt bath to T=-5°C-5°C, add NBS (19.4g, 108.7mmol, 0.9eq), add After the reaction was completed for 0.5h, water was added to quench the reaction, extracted with ethyl acetate (200ml*2), the organic layers were combined, washed with water, and concentrated under reduced pressure to ...
Embodiment 3
[0042] a) Add compound 1 (50.0g, 204.1mmol) in a 1000ml three-necked flask, add isopropyl acetate (400ml), add triethylamine (24.7g, 245.0mmol, 1.2eq), drop to internal temperature T=-10 ℃--5℃, dropwise add pivaloyl chloride (25.0g, 122.5mmol, 1eq), after the dropwise addition is complete, T=-5℃-10℃ for 4h, then add 100ml of isopropyl acetate solution of compound 2 (23.2 g, 214mmol, 1.05eq), after adding, keep warm for one hour, slowly return to room temperature and react for 8h, add water to quench, extract with isopropyl acetate (300ml*2), combine the organic layers, and use 1N HCl aqueous solution ( 100ml), washed with water (100ml), the organic phase was concentrated under reduced pressure to obtain the crude product of compound 3, the crude product of compound 3 was dissolved by adding 5 times the mass of isopropyl acetate, heated and dissolved, concentrated to 1 times the volume, added 4 times the volume of petroleum ether to crystallize, Compound 3 was obtained, 54 g (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com