Keggin-structure heteropolyacid phosphorus-containing organic salt compound as well as preparation method and application
A technology of compound and heteropolyacid, which is applied in chemical synthesis and pharmaceutical application fields, to achieve the effect of high safety, low cytotoxicity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 (preparation of PZ-1)
[0018] Dissolve 91 mmol of sodium tungstate dihydrate and 43 mmol of sodium dihydrogen phosphate in 100 mL of distilled water, add 16 mmol of titanium tetrachloride dropwise, reflux for 30 minutes, filter, add 4.1 mol of solid KCl to the filtrate to obtain a white precipitate, and use Hot water recrystallization gave solid K 7 PTi 2 W 10 o 40 . 10mmol K 7 PTi 2 W 10 o 40 Dissolve in 100mL water, add 100mmol tenofovir, adjust the pH value to 4.0, heat to 60°C, react for 3h, after standing for a while, a white solid (C 9 h 15 N 5 PO 4 ) 7 PTi 2 W 10 o 40 .
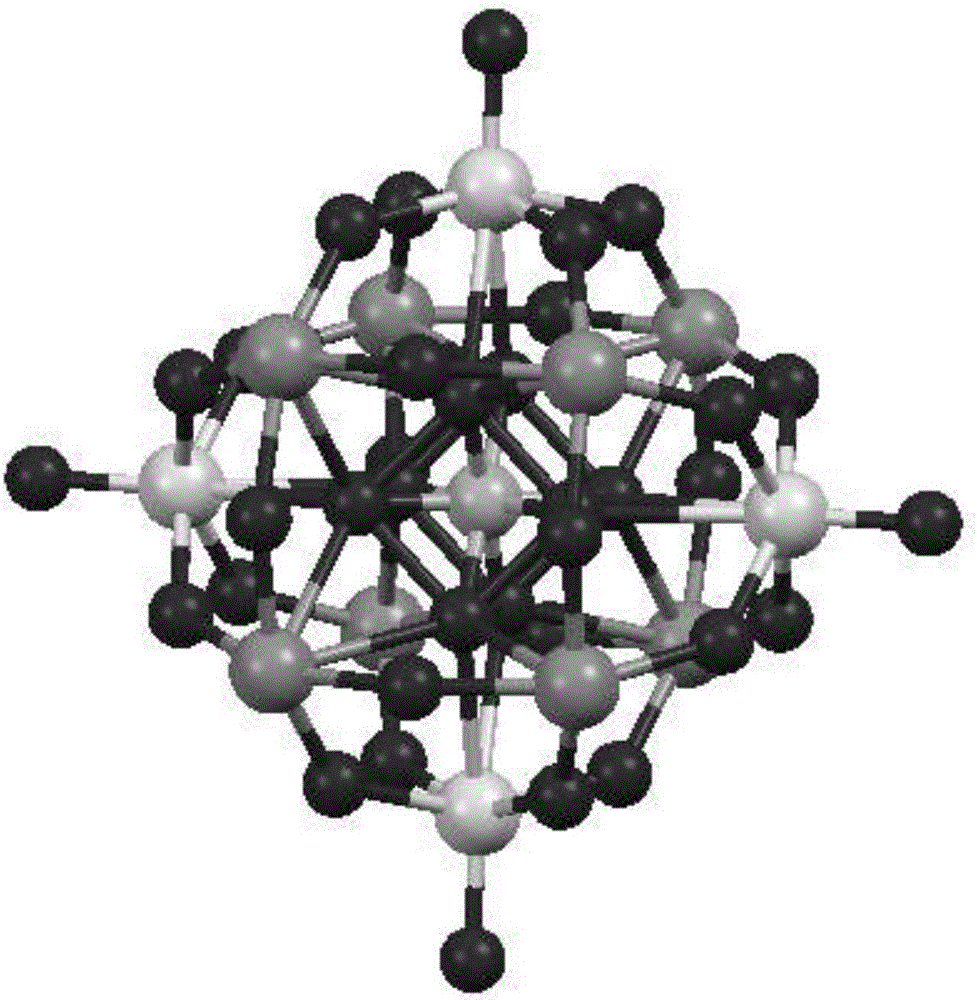
[0019] 1 H NMR (400MHz, DMSO-d 6 ):δ H (ppm)1.020(d,3H),3.590(m,2H),3.909(m,1H),4.255(m,2H),8.133(s,1H),8.147(s,1H),9.271(s,3H ), 10.960 (s, 2H). Elemental analysis (4622.24): calcd (%): C, 16.36; N, 10.61; P, 5.36; Ti, 2.07; W, 39.76; found (%): C, 16.43; N ,10.68; P,5.33; Ti,2.02; W,39.69.PTi 2 W 10 o 40 7- X-ray single crystal diffraction structure diagr...
Embodiment 2
[0020] Embodiment 2 (preparation of PZ-2)
[0021] Dissolve 91 mmol of sodium tungstate dihydrate and 43 mmol of sodium dihydrogen phosphate in 100 mL of distilled water, add 16 mmol of titanium tetrachloride dropwise, reflux for 30 minutes, filter, add 4.1 mol of solid KCl to the filtrate to obtain a white precipitate, and use Hot water recrystallization gave solid K 7 PTi 2 W 10 o 40 . 10mmol K 7 PTi 2 W 10 o 40 Dissolve in 100mL water, add 100mmol adefovir, adjust the pH value to 4.0, heat to 60°C, react for 3h, after standing for a while, a white solid (C 8 h 13 N 5 PO 4 ) 7 PTi 2 W 10 o 40 .
[0022] 1 H NMR (400MHz, DMSO-d 6 ):δ H(ppm)3.260(s,2H),3.729(t,2H),4.195(t,2H),7.957(s,1H),8.021(s,1H),9.160(s,3H),10.754(s,2H ). Elemental analysis (4524.10): calcd (%): C, 14.85; N, 10.83; P, 5.48; Ti, 2.12; W, 40.63; found (%): C, 14.95; N, 10.89; Ti, 2.16; W, 40.56.PTi 2 W 10 o 40 7- X-ray single crystal diffraction structure diagram figure 1 , the basi...
Embodiment 3
[0023] Embodiment 3 (preparation of PZ-3)
[0024] Dissolve 91 mmol of sodium tungstate dihydrate and 43 mmol of sodium dihydrogen phosphate in 100 mL of distilled water, add 16 mmol of titanium tetrachloride dropwise, reflux for 30 minutes, filter, add 4.1 mol of solid KCl to the filtrate to obtain a white precipitate, and use Hot water recrystallization gave solid K 7 PTi 2 W 10 o 40 . 10mmol K 7 PTi 2 W 10 o 40 Dissolve in 100mL water, add 100mmol diethyl (α-aminobenzyl) phosphonate hydrochloride, adjust the pH value to 7.0, heat to 60°C, react for 3h, after standing for a while, a white solid (C 11 h 19 NPO 3 ) 7 PTi 2 W 10 o 40 .
[0025] 1 H NMR (400MHz, DMSO-d 6 ):δ H (ppm)1.090(t,3H),1.245(t,3H),3.821-4.116(m,4H),4.939(d,1H),7.412-7.569(m,5H),9.093(s,3H). Analysis (4314.52): calcd (%): C, 21.42; N, 2.27; P, 5.74; Ti, 2.22; W, 42.60; found (%): C, 21.53; N, 2.32; ;W,42.53.PTi 2 W 10 o 40 7- X-ray single crystal diffraction structure diagram figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com