Luminous metal organic frame compound and preparing method and application thereof
A technology of metal-organic frameworks and compounds, which is applied in the fields of solvent stability, metal-organic framework compounds and their preparation, acid and alkali resistance and fluorescence characteristics, and can solve problems such as limiting the application range of luminescent materials, reducing luminous efficiency, and aggregation quenching , achieving good solvent stability and acid and alkali resistance, simple synthesis method, and overcoming poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
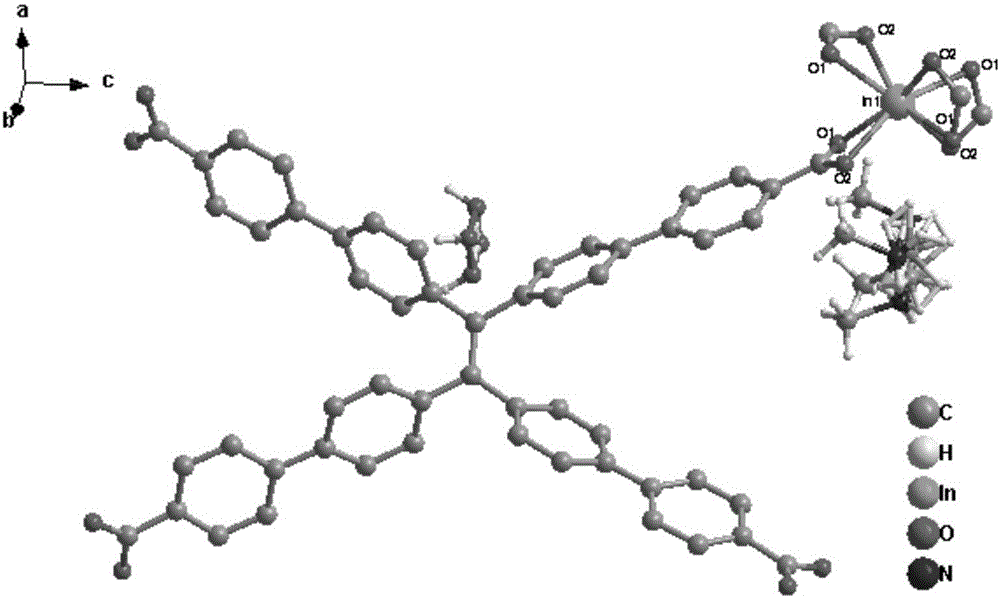
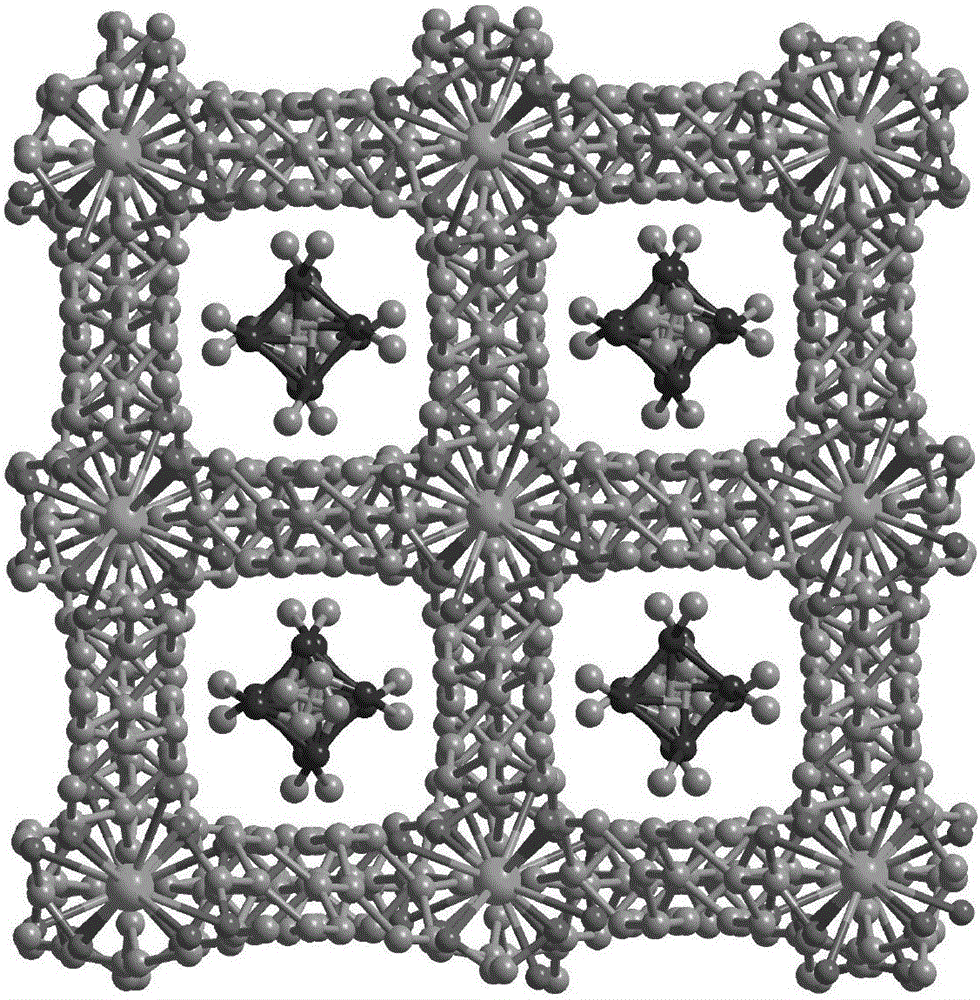
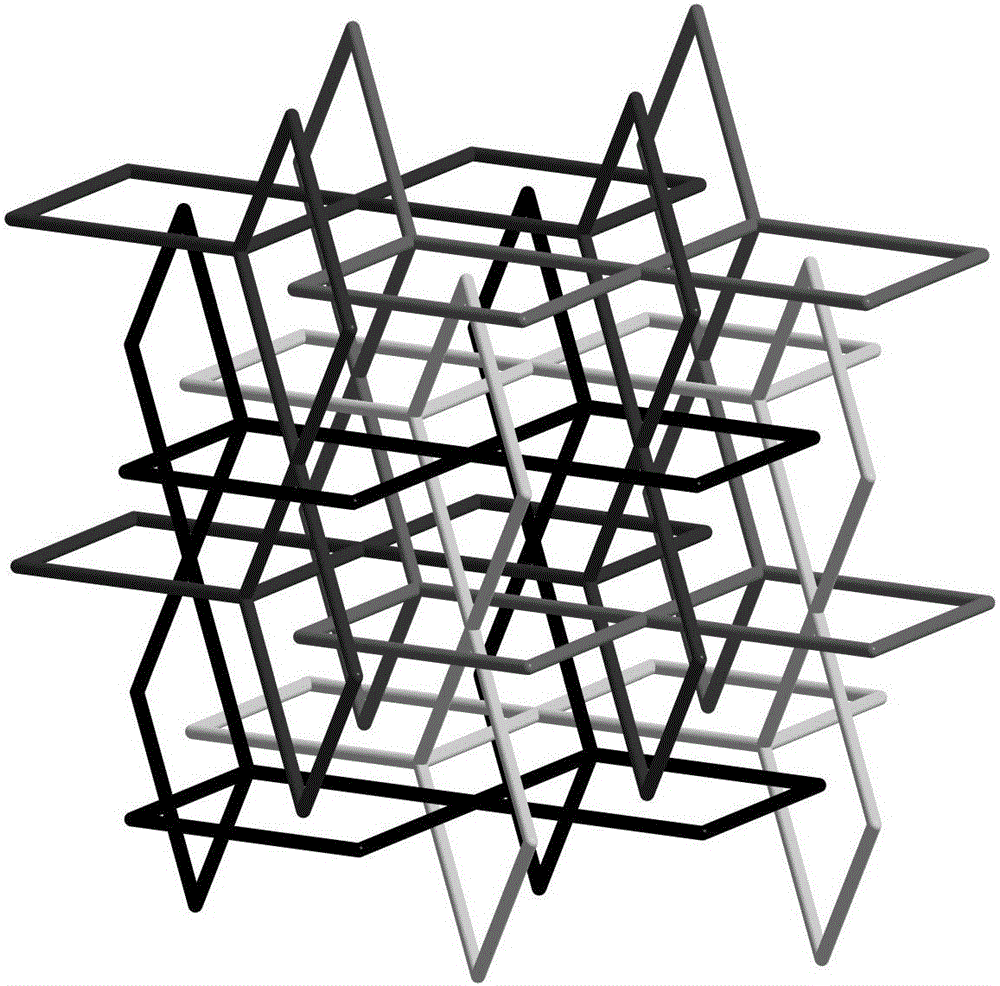
[0037] Compound [In(tcbpe)][(CH 3 ) 2 NH 2 ]·H 2 Synthesis of O(MOF-In)(MOF--In)
[0038] Accurately weigh indium nitrate (0.02mmol), 1,1,2,2-tetrakis[(4-carboxyphenyl)benzene]ethylene (0.015mmol) into a 25mL high temperature resistant glass bottle, then add 2mL N,N - Dimethylformamide and 1mL acetonitrile, ultrasonically dissolved, then dropwise added 0.5mL dilute nitric acid (V 水 :V 浓硝酸 =3:2), the solution became clear. Put the glass bottle in a constant temperature drying oven, react at a constant temperature at 100°C for 48 hours, then cool down naturally to obtain yellow blocky crystals, and filter to obtain the product. Yield: 40%, Infrared (IR): 3435(m), 3045(m), 1601(s), 1525(s), 1392(s), 1012(s), 783(s).
Embodiment 2
[0040] Compound [Eu(tcbpe)][(CH 3 ) 2 NH 2 ]·H 2 Synthesis of O(MOF-Eu)(MOF--Eu)
[0041] Accurately weigh europium nitrate (0.02mmol), 1,1,2,2-tetrakis[(4-carboxyphenyl)benzene]ethylene (0.015mmol) into a 25mL high temperature resistant glass bottle, then add 2mL N,N - Dimethylformamide and 1mL acetonitrile, ultrasonically dissolved, then dropwise added 0.5mL dilute nitric acid (V 水 :V 浓硝酸 =3:2), the solution became clear. Put the glass bottle in a constant temperature drying oven, react at a constant temperature at 100°C for 48 hours, then cool down naturally to obtain yellow blocky crystals, and filter to obtain the product. Yield: 46%, Infrared (IR): 3412(m), 3064(m), 1601(s), 1525(s), 1420(s), 1002(s), 850(s), 793(s) .
Embodiment 3
[0043] Compound [Gd(tcbpe)][(CH 3 ) 2 NH 2 ]·H 2 Synthesis of O(MOF-Gd)(MOF--Gd)
[0044] Accurately weigh gadolinium nitrate (0.02mmol), 1,1,2,2-tetrakis[(4-carboxyphenyl)benzene]ethylene (0.015mmol) into a 25mL high temperature resistant glass bottle, then add 2mL N,N - Dimethylformamide and 1mL acetonitrile, ultrasonically dissolved, then dropwise added 0.5mL dilute nitric acid (V 水 :V 浓硝酸 =3:2), the solution became clear. Put the glass bottle in a constant temperature drying oven, react at a constant temperature at 100°C for 48 hours, then cool down naturally to obtain yellow blocky crystals, and filter to obtain the product. Yield: 45%. Infrared (IR): 3416(m), 3064(m), 1592(s), 1525(s), 1430(s), 1002(s), 859(s), 793(s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com