Method for preparing chiral (S)-acetoin by virtue of whole-cell biological catalysis
A technology of biocatalysis and biocatalyst, which is applied in the direction of biochemical equipment and methods, methods based on microorganisms, microorganisms, etc., can solve the problems of cost reduction, achieve the effect of improving regeneration ability, simple operation process, and high-tech economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
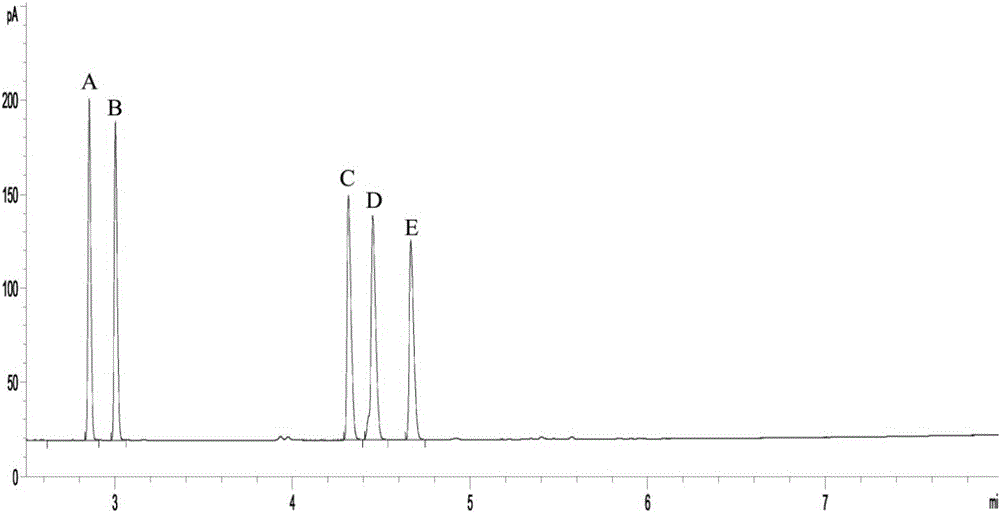
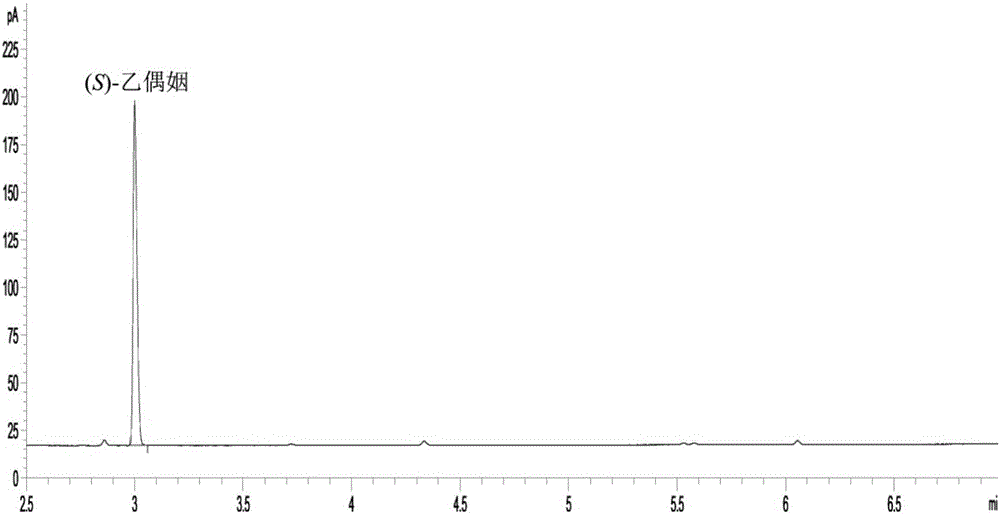
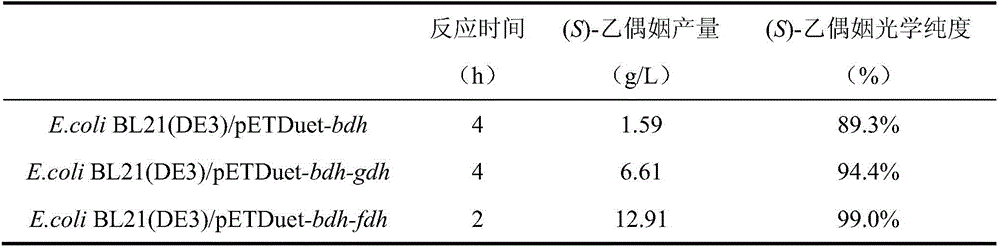
[0035] This implementation confirms that the new method of co-expression of formate dehydrogenase and diacetyl reductase to produce (S)-acetoin of the present invention is superior to co-expression of glucose dehydrogenase in terms of production efficiency, yield and optical purity through comparative experiments system.
[0036] 1. Preparation of recombinant Escherichia coli
[0037] The formate dehydrogenase gene fdh from Saccharomyces cerevisiae and the glucose dehydrogenase gene gdh from B. subtilis168 were artificially synthesized after codon optimization, and cloned into the expression vector pETDuet-1 to obtain a recombinant plasmid pETDuet-fdh and pETDuet-gdh. The diacetyl reductase gene bdh from Enterobacter cloacae was artificially synthesized after codon optimization, and cloned into the plasmids pETDuet-1, pETDuet-gdh and pETDuet-fdh respectively to obtain the recombinant plasmids pETDuet-bdh and pETDuet -bdh-gdh and pETDuet-bdh-fdh, which can be transformed into...
Embodiment 2
[0051] 1. Preparation of recombinant Escherichia coli
[0052] The diacetyl reductase gene adr derived from Rhodococcus erythropolis was artificially synthesized after codon optimization, and cloned into the recombinant plasmid pETDuet-fdh obtained in Example 1 to obtain the recombinant plasmid pETDuet-adr-fdh. E. coli E. coli BL21 (DE3) competent cells were transformed to obtain recombinant E. coli E. coli BL21 (DE3) / pETDuet-adr-fdh. The nucleotide sequence of the artificially synthesized codon-optimized Rhodococcus erythropolis diacetyl reductase gene adr is shown in SEQ ID NO.4.
[0053] 2. The preparation of recombinant Escherichia coli resting cells comprises the following steps:
[0054] (1) Plate culture: Streak the recombinant E. coli E. coli BL21(DE3) / pETDuet-adr-fdh on the LB plate medium containing 1.8% agar by mass volume ratio and 100 μg / mL ampicillin, at 37°C Overnight culture;
[0055] (2) Seed culture: under sterile ultra-clean bench conditions, pick a singl...
Embodiment 8
[0065] 1. Preparation of recombinant Escherichia coli
[0066] The formate dehydrogenase gene fdh from Saccharomyces cerevisiae was artificially synthesized after codon optimization, and cloned into the expression vector pETDuet-1 to obtain the recombinant plasmid pETDuet-fdh. The diacetyl reductase gene bdh derived from Enterobacter cloacae was artificially synthesized after codon optimization, and cloned into the plasmid pETDuet-fdh to obtain the recombinant plasmid pETDuet-bdh-fdh, which was then transformed into Escherichia coli E .coliBL21(DE3) competent cells can be obtained from recombinant Escherichia coli E.coli BL21(DE3) / pETDuet-bdh-fdh. The nucleotide sequences of the artificially synthesized codon-optimized formate dehydrogenase gene fdh and the diacetyl reductase gene bdh are shown in SEQ ID NO.1 and SEQ ID NO.3.
[0067] 2. The preparation of recombinant Escherichia coli resting cells comprises the following steps:
[0068] (1) Plate culture: Streak the recombi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com