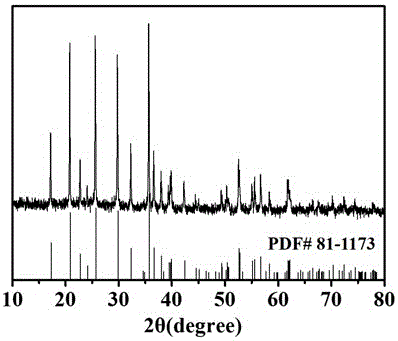
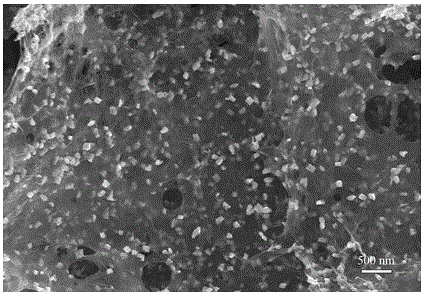
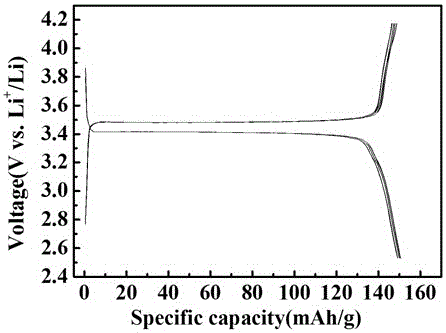
Method for preparing graphene/lithium iron phosphate composite anode materials
A composite positive electrode material, lithium iron phosphate technology, applied in the field of materials science, can solve problems such as poor electrochemical performance, and achieve the effects of excellent electrochemical performance, good electrical conductivity and electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A preparation method of graphene / lithium iron phosphate composite positive electrode material, the raw materials required for its preparation are calculated in parts by mass, and its composition and consumption are as follows:
[0022] 7.6 parts of lithium hydroxide;
[0023] 50 parts of ferrous sulfate;
[0024] 20.7 parts of phosphoric acid;
[0025] 4000 parts of 3mg / mL graphene oxide solution;
[0026] 14.2 parts of ethylenediamine;
[0027] Firstly, lithium hydroxide, ferrous sulfate and phosphoric acid are dissolved in the graphene oxide solution, then ethylenediamine is added, and stirred evenly. Then, the mixed solution was placed in a polytetrafluoroethylene reactor for hydrothermal reaction at 220° C. for 12 hours. During the hydrothermal reaction process, under the catalysis of ethylenediamine, lithium iron phosphate particles are reacted, and graphene oxide microsheets self-assemble and the precipitated lithium iron phosphate particles are tightly wrappe...
Embodiment 2
[0032] A preparation method of graphene / lithium iron phosphate composite positive electrode material, the raw materials required for its preparation are calculated in parts by mass, and its composition and consumption are as follows:
[0033] 14.5 parts of lithium nitrate;
[0034] 84.8 parts of ferric nitrate;
[0035] Phosphoric acid 24.2 parts;
[0036] 4000 parts of 3mg / mL graphene oxide solution;
[0037] 16.6 parts of ethylenediamine;
[0038] First, lithium nitrate, iron nitrate and phosphoric acid are dissolved in the graphene oxide solution, then ethylenediamine is added, and stirred evenly. Then the mixed solution was placed in a polytetrafluoroethylene reactor for hydrothermal reaction at 160° C. for 8 hours. During the hydrothermal reaction process, under the catalysis of ethylenediamine, lithium iron phosphate particles are reacted, and graphene oxide microsheets self-assemble and the precipitated lithium iron phosphate particles are tightly wrapped between the ...
Embodiment 3
[0043] A preparation method of graphene / lithium iron phosphate composite positive electrode material, the raw materials required for its preparation are calculated in parts by mass, and its composition and consumption are as follows:
[0044] 16.5 parts of lithium acetate;
[0045] 43.5 parts of ferrous acetate;
[0046] 28.8 parts of phosphoric acid;
[0047] 4000 parts of 3mg / mL graphene oxide solution;
[0048] 19.4 parts of ethylenediamine;
[0049] First, dissolve lithium acetate, ferrous acetate and phosphoric acid in the graphene oxide solution, then add ethylenediamine, and stir evenly. Then, the mixed solution was placed in a polytetrafluoroethylene reactor for hydrothermal reaction at 160° C. for 16 hours. During the hydrothermal reaction process, under the catalysis of ethylenediamine, lithium iron phosphate particles are reacted, and graphene oxide microsheets self-assemble and the precipitated lithium iron phosphate particles are tightly wrapped between the se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com