Garnet-type Li-ion conductive oxide
A garnet-type, lithium-ion technology, used in electrochemical generators, electrolyte immobilization/gelling, electrical components, etc., can solve the problems of high reactivity, low lithium metal potential, and no solid electrolyte, and achieve high Effects of ionic conductivity and high sintered density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
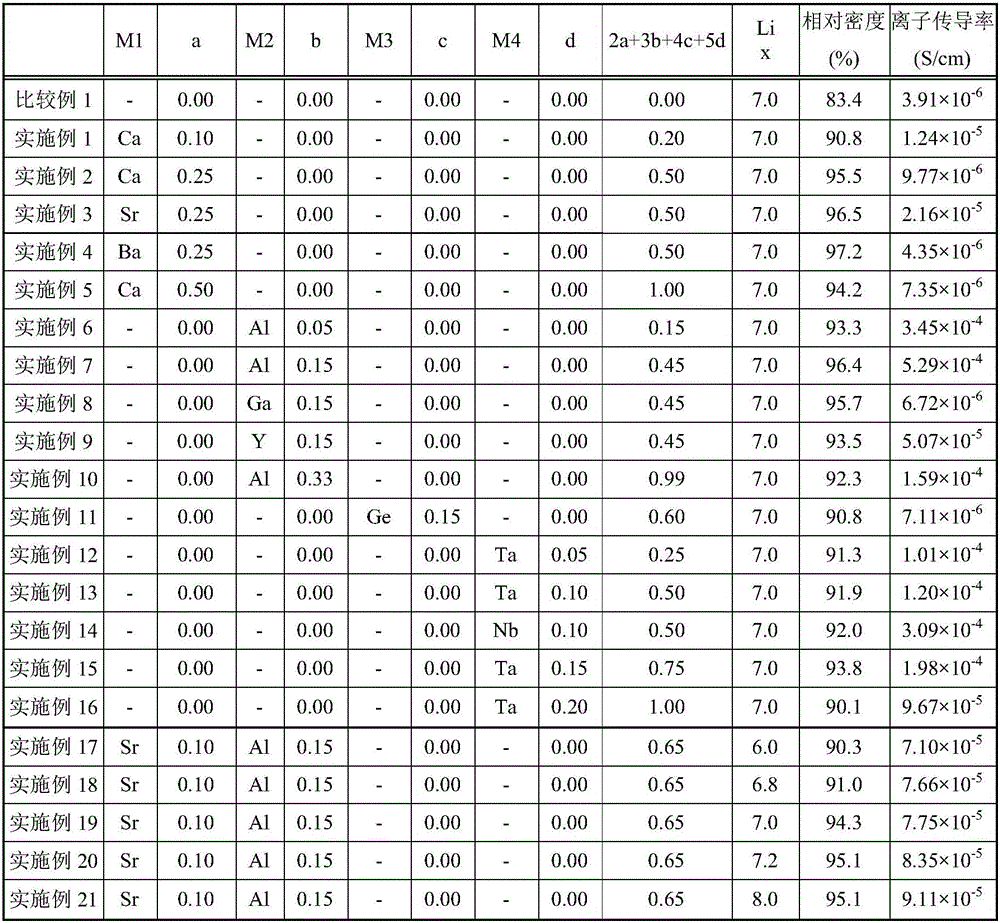
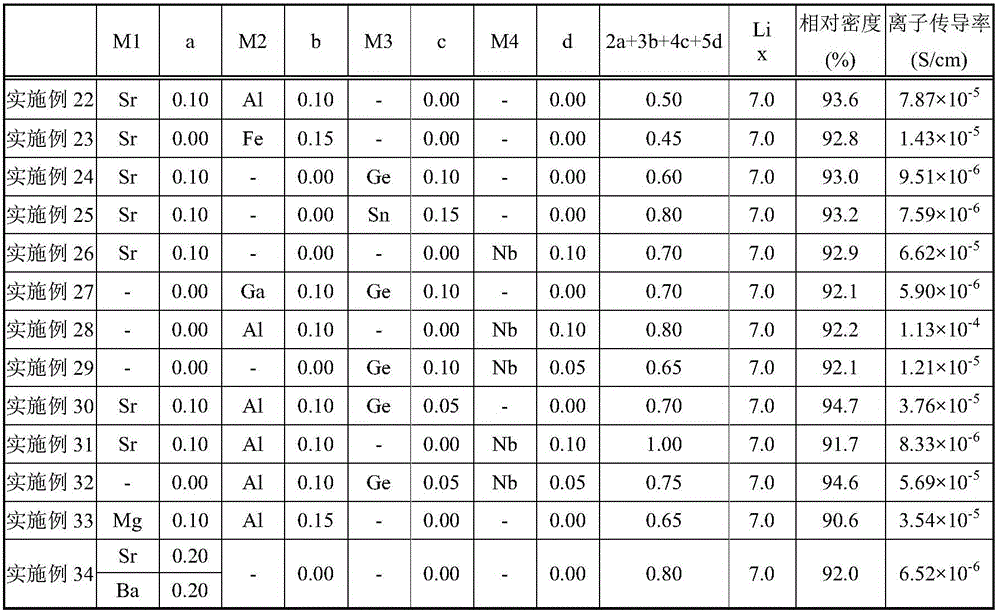
[0129] The content of the present invention will be described more specifically with reference to Examples and Comparative Examples, but the present invention is not limited to the following Examples.
[0130] As Examples 1-21, the composition formula Li of the garnet-type lithium ion conductive oxide is x-2a-3b-4c-5d M1 a M2 b M3 c M4 d La 3 Zr 2 o 12 , M1, a, M2, b, M3, c, M4, and d are as described in Table 1. The raw materials corresponding to the respective elements were weighed and mixed. As a raw material, LiCO is used 3 、La(OH) 3 , ZrO 2 , MgCO 3 , CaCO 3 , SrCO 3 、BaCO 3 、Al 2 o 3 , Ga 2 o 3 , Y 2 o 3 、GeO 2 、 Ta 2 o 5 , Nb 2 o 5 . Mixing was performed using ethanol solvent and using a ball mill (120 rpm / zirconia ball) for 16 hours.
[0131] Then, the obtained slurry was separated and dried from ethanol and zirconia balls to obtain a mixed powder.
[0132] Next, the obtained mixed powder was placed in a MgO container, and calcined at 900° C....
Embodiment 35
[0156] Examples of all-solid lithium secondary batteries are shown below, but the present invention is not limited to these examples. In addition, "part" means a mass part unless otherwise indicated.
[0157] (Production of positive electrode active material and negative electrode active material)
[0158] As the positive electrode active material and the negative electrode active material, Li 3 V 2 (PO 4 ) 3 . As the production method, with Li 2 CO 3 , V 2 o 5 , NH 4 h 2 PO 4 As the starting material, it was wet-mixed with a ball mill for 16 hours, and after dehydration and drying, the obtained powder was calcined at 850° C. in nitrogen-hydrogen mixed gas for two hours. The calcined product is wet pulverized by a ball mill, and then dehydrated and dried to obtain a powder. Using an X-ray diffraction device, it was confirmed that the structure of the produced powder was Li 3 V 2 (PO 4 ) 3 .
[0159] (Preparation of positive electrode active material slurry an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com