Deferoxamine derivative compound with bone affinity, preparation method and application thereof
A compound and hydrate technology, applied in the direction of medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve the problem of increasing DFO dose and medication time, limiting the effect of DFO bone action, slow subcutaneous or intravenous infusion And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
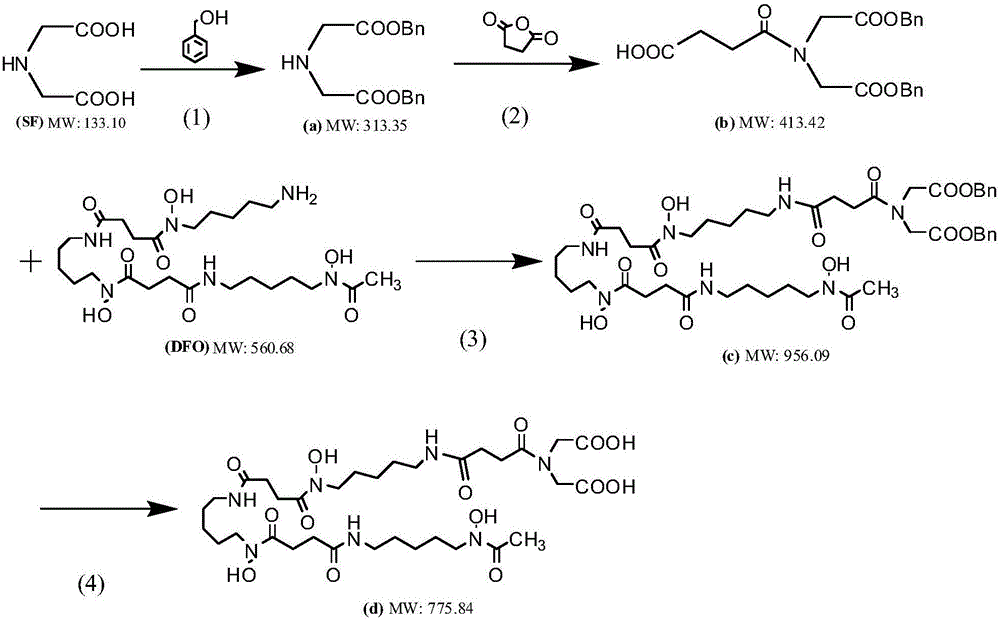
[0077] Desferoxamine, deferoxamine derivative compound a, deferoxamine derivative compound b, and defersoxamine derivative compound c used in the following synthesis examples 1-4 were all purchased from Sigma; iminodiacetic acid and 2,2'- Hydrazine-1,2-diyldiacetic acid, 2,2'-methylene-diureadiyldiacetic acid, 2,2'-propyl-1,3-diureadiyldiacetic acid were purchased from Aladdin Company; succinic anhydride and adipic anhydride were purchased from Sinopharm and Aladdin respectively; PyBOP was purchased from Sigma; Pd / C was purchased from Sigma, and its Pd doping amount was 10%; the rest of the chemical reagents were purchased from Sigma The company's analytical grade reagents; the water used is deionized double distilled water. The reaction is carried out under normal temperature and pressure conditions. The melting points of the compounds were determined with a Fisher-John melting point apparatus. NMR spectra were measured with a Bruker AM-400 spectrometer. The mass spectrum ...
Embodiment 5
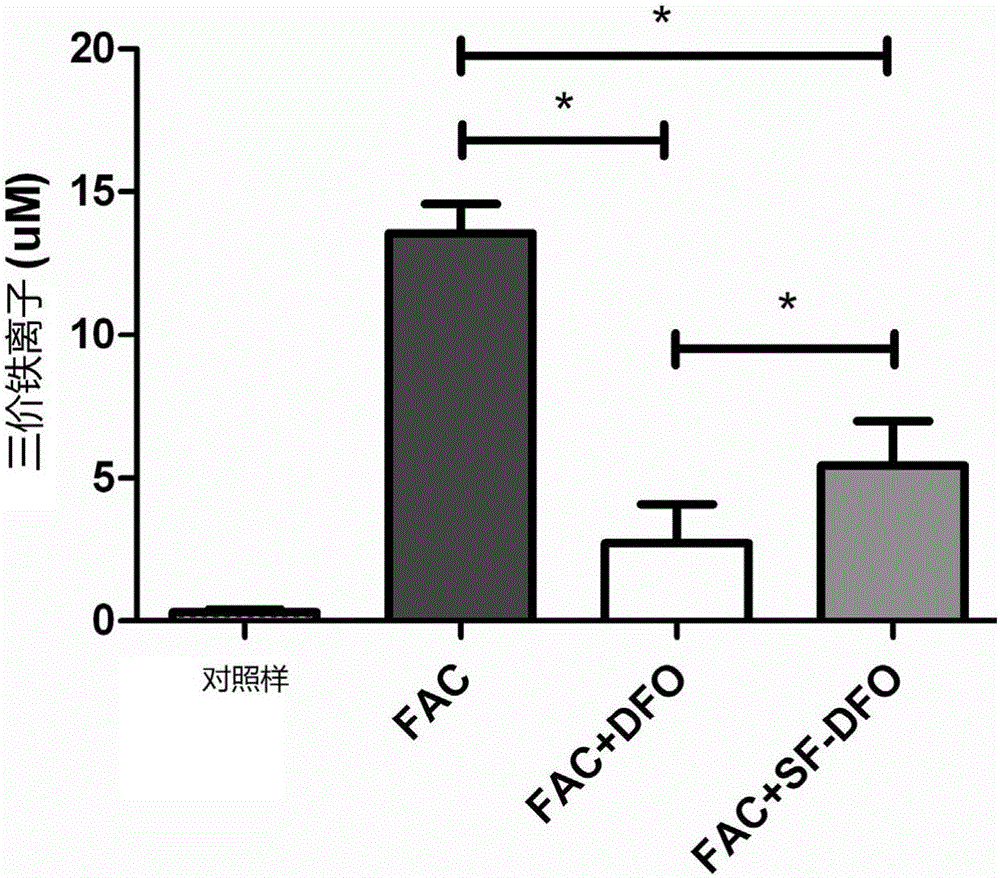
[0098] Embodiment 5 Ferric ion complex performance test
[0099] In this example, the complexing ability of the compounds prepared in the above examples to ferric ions was characterized. Specifically, ferric ammonium citrate (FAC) was used as a ferric ion donor, dissolved in double distilled water to form a solution, and the same molar amounts of the compounds prepared in the above synthesis examples 1-4 were added thereto As well as deferoxamine that has not been substituted and modified as described in the present invention, use a Beckman DXC600 automatic biochemical analyzer to detect the content of free iron ions in the solution to know whether the compound of the present invention has good characteristics of complexing iron ions. figure 2 The DFO-SF prepared in Example 1 and the unsubstituted DFO are shown to be complexed to iron ions, figure 2 The control in is the iron ion content measured using only double distilled water without adding FAC. The compounds prepared ...
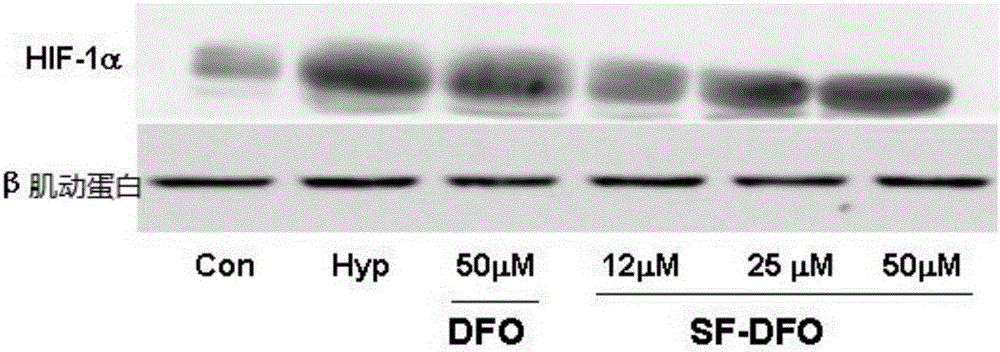
Embodiment 6
[0101] Embodiment 6 Stimulates the performance of cells expressing hypoxia-inducible factor (HIF)-α and its downstream target genes
[0102] In this example, the ability of the compounds of the present invention to stimulate cells to express hypoxia-inducible factor (HIF)-α and its downstream target genes was investigated. Specifically, the mouse calvaria 48 hours after birth was removed and cut into 1×1×1mm 3 Small pieces, digested with trypsin and type Ⅰ collagenase, isolated and purified newborn mouse calvarial osteoblasts; 2×10 4 / cm 2 The cell density was inoculated to a 6-well culture plate; when the cells adhered to the bottom of the culture plate and grew to 80% mixed, the conditioned medium with a compound concentration of 200 μM (DFO net concentration) of the embodiments of the present invention was used respectively for the above all The newborn mouse calvarial osteoblasts were cultured, and the conditioned medium with a concentration of 200 μM of unsubstituted mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com