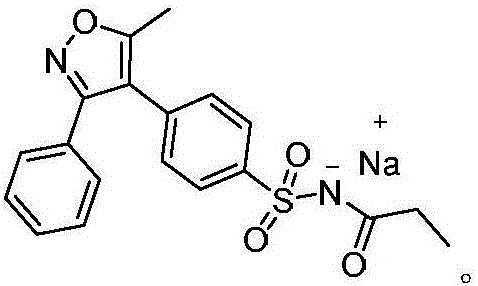
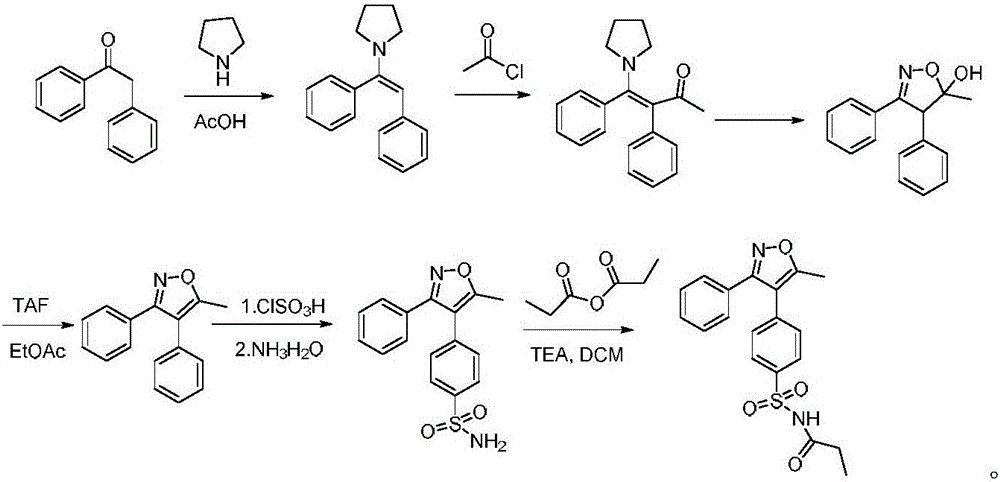
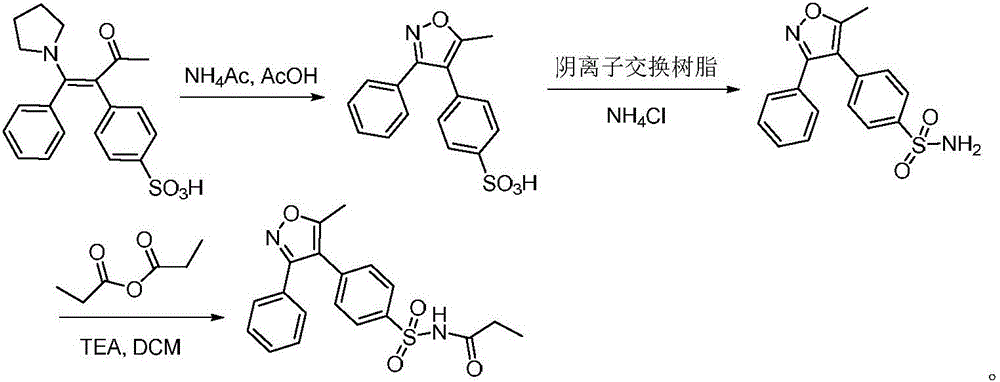
Preparation method of parecoxib for treating postoperative pain
A parecoxib and phenyl technology, applied in the field of drug synthesis, can solve the problems of low overall yield, high equipment requirements, environmental pollution, etc., and achieve the effect of simple reaction treatment, simple reaction steps, and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0027] Add 37.1 g (100 mmol) of 3-(4-sulfophenyl)-4-phenyl-4-(1-pyrrolidinyl)-3-buten-2-one and 38 g (500 mmol) of ammonium acetate to In a flask containing 65ml of acetic acid, contact reaction at 95°C for 0.5 hours. After the reaction, dilute with dichloromethane, adjust the pH to 7 with saturated sodium bicarbonate, concentrate the organic phase, wash with water, then recrystallize with ethanol, and dry to obtain 5-methyl -26.8 g of 3-phenyl-4-(4-sulfophenyl)isoxazole, the yield is 85.0%, and the purity is 99.77%.
Embodiment 2
[0029] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0030] Add 37.1 g (100 mmol) of 3-(4-sulfonic acid phenyl)-4-phenyl-4-(1-pyrrolidinyl)-3-buten-2-one and 30 g (400 mmol) of ammonium acetate to In a flask with 70ml of acetic acid, contact reaction at 100°C for 1 hour. After the reaction, dilute with dichloromethane, adjust the pH to 7 with saturated sodium bicarbonate, concentrate the organic phase, wash with water, then recrystallize with ethanol, and dry to obtain 5-methyl - 26.9 g of 3-phenyl-4-(4-sulfophenyl)isoxazole, the yield was 85.2%, and the purity was 99.84%.
Embodiment 3
[0032] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0033] Add 37.1 g (100 mmol) of 3-(4-sulfonic acid phenyl)-4-phenyl-4-(1-pyrrolidinyl)-3-buten-2-one and 30 g (400 mmol) of ammonium acetate to In a flask containing 90ml of acetic acid, contact reaction at 90°C for 1.5 hours. After the reaction, dilute with dichloromethane, adjust the pH to 6 with saturated sodium bicarbonate, concentrate the organic phase, wash with water, then recrystallize with ethanol, and dry to obtain 5-methyl - 26.8 g of 3-phenyl-4-(4-sulfophenyl)isoxazole, the yield was 84.9%, and the purity was 99.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com