A kind of preparation method of Trexagliptin
A compound, methyl technology, applied in the field of preparation of trexagliptin, can solve the problem of low purity of trexagliptin, and achieve the effects of high conversion rate, mild reaction conditions and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
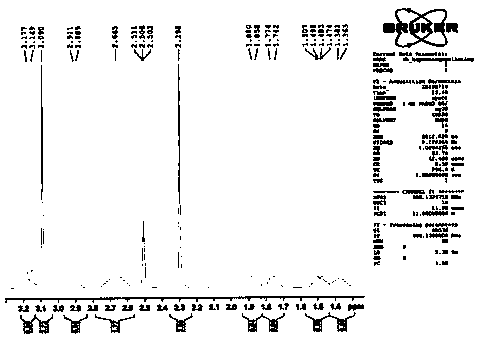
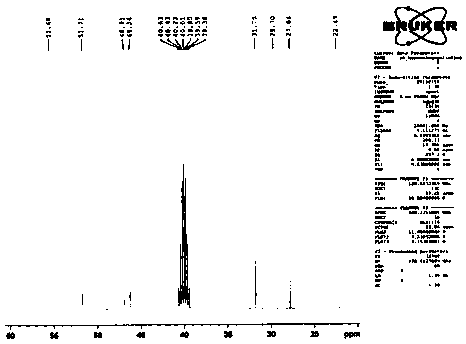
[0048] Example 1: Preparation of compound shown in formula 4
[0049] The compound shown in formula 6 (100g, 340.5mmol), the compound shown in formula 5 (49.0g, 375mmol) and potassium carbonate (94.0g, 682.0mmol) were added in N,N-dimethylformamide (600ml), Raise the temperature to 70°C and react for 2 hours. After the reaction, cool down to 20°C. Add 1800ml of water and 2M / L dilute hydrochloric acid to adjust the pH of the feed solution to ≤ 6. A large amount of solids are precipitated. After stirring for 30 minutes, filter, rinse with 100ml of ethanol, and dry to obtain Off-white compound 130.2g, purity 99.7%, maximum single impurity 0.1%. The yield was 98.9%.
[0050] ESI-MS: m / z([M+H] + ) is 387.4.
example 2
[0051] Example 2: step2-1, the preparation of compound (X is Cl) shown in formula 3-1
[0052] The compound shown in formula 4 (5g, 12.94mmol) was added in 50ml of dichloromethane, and the temperature was lowered to 0°C, and thionyl chloride (1.8g, 15mmol) was added dropwise. Concentrate under reduced pressure to dryness to obtain 4.9 g of a light yellow solid, with a yield of 93.5%, a purity of 98.7%, and a maximum single impurity of 0.1%.
[0053] ESI-MS: m / z([M+H] + ) is 405.8.
example 3
[0054] Example 3: step2-1, the preparation of compound (X is Br) shown in formula 3-1
[0055] Add the compound shown in Formula 4 (5g, 12.94mmol) into 50ml of dichloromethane, drop the temperature to 0°C, and add a solution of phosphorus oxybromide (4.3g, 15.0mmol) dropwise in dichloromethane, and continue the reaction for 1 hour after dropping , heated to 30° C. and concentrated under reduced pressure to dryness to obtain 5.5 g of a light yellow solid with a yield of 94.6%, a purity of 98.4%, and a maximum single impurity of 0.13%.
[0056] ESI-MS: m / z([M+H] + ) is 450.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com