A kind of organic polymer, its preparation method and application
A polymer and organic technology, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as limiting industrial application and promotion, device manufacturing process and stability, and achieve good environmental stability and optoelectronics properties, favorable charge transport, and good solution processing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
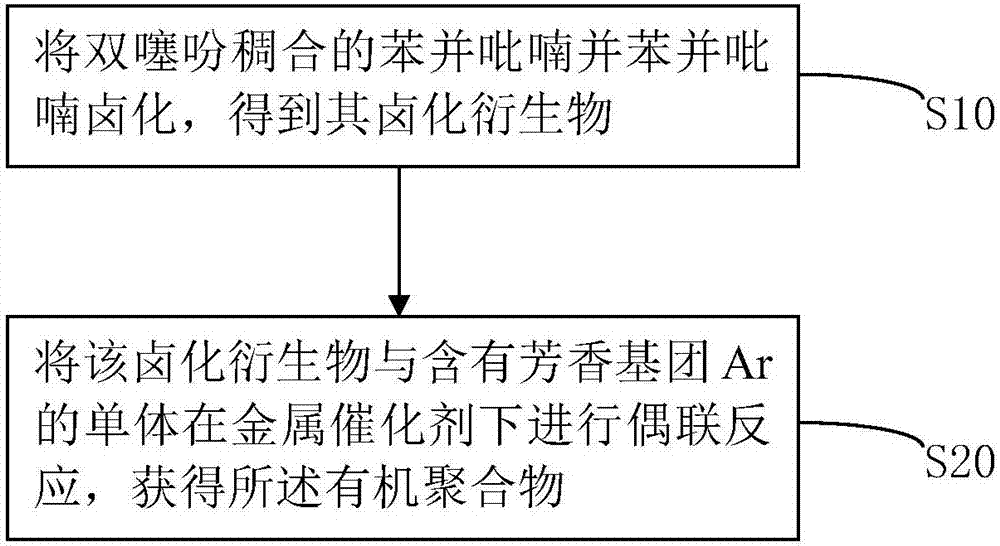
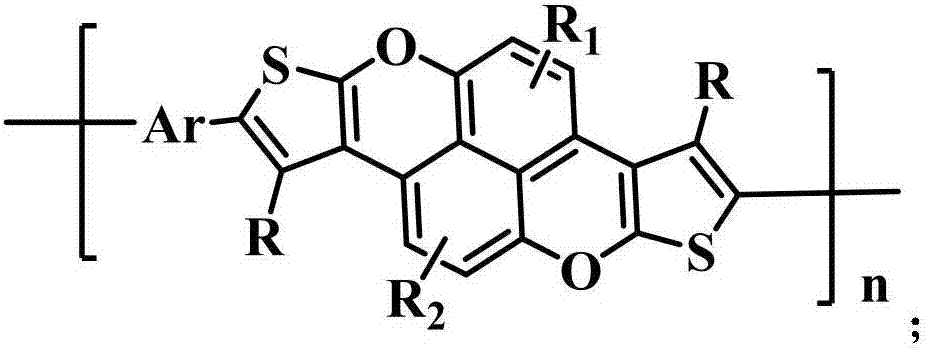
[0049] Such as figure 1 As shown, the preparation method of the aforementioned organic polymer includes: S10, halogenating the benzopyranochromene condensed with dithiophene to obtain its halogenated derivative; S20, combining the halogenated derivative with an aromatic group Ar The monomer is subjected to a coupling reaction under a metal catalyst to obtain the organic polymer. Wherein, after the coupling reaction, the aromatic group Ar is connected with the dithiophene-fused benzopyranochromene unit through conjugation.
[0050] Wherein, the synthesis of dithiophene-fused benzopyranochromene can refer to Chinese patent publication CN103497758A, the entire disclosure content of which is incorporated herein by reference. The foregoing coupling reactions include, but are not limited to, Suzuki coupling, Stille coupling, Negishi coupling, Hiyama coupling, C-H activated direct coupling, and the like.
[0051] As one of the schemes that can be implemented, the method includes bu...
Embodiment 1
[0056] The preparation of the organic polymer provided in this example mainly includes two parts: the preparation of the monomer and the polymerization reaction. First, the synthesis of dithiophene-fused benzopyranochromene can be prepared with reference to Chinese patent publication No. 103497758A, 2,7-bis(4,4,5,5-tetramethyl-1,3 , 2-dioxaborinane-diyl)-N-9-heptadecylcarbazole according to literature (N.Blouin, A.Michaud, M.Leclerc, Adv.Mater., 2007,19,2295-2300 )preparation.
[0057] Specifically, monomer 1 (dithiophene-fused benzopyranochromene) and polymer P 1 The synthetic route of (target product) is as follows:
[0058]
[0059]
[0060] 1. Synthesis of monomer 1:
[0061] Concrete reaction condition is: (1) Br 2 , CS 2 , reflux, 24h. (II)CH 3 COOH, Zn, reflux, 2h. (Ⅲ)C 8 h 17 MgBr, [NiCl 2 (dppp)], Et 2 O, reflux, 12h. (Ⅳ)Br 2 , CH 2 Cl 2 , rt, 2h.
[0062] More specifically:
[0063] Step (I): In the carbon disulfide solution (40mL) of benzopyr...
Embodiment 2
[0070] The synthetic route of the organic polymer in the present embodiment is as follows:
[0071]
[0072] One, the synthesis of monomer 1 is the same as in Example 1, another monomer 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)- Behenyl fluorene can be purchased directly.
[0073] 2. Polymer P 2 Synthesis:
[0074] 377mg (0.5mmol) 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-dioctylfluorene, 350mg (0.5mmol ) monomer 1,6mgPd 2 (dba) 3 , 12mg of P(o-tol) 3 Add it to a 25m1 two-necked bottle, pump and change gas three times until the hydrogen is full, add 4m120% Et with a syringe 4 NOHI organic base solution and 10mL toluene, reflux reaction for 72 hours, add phenylboronic acid and bromobenzene to block respectively, drop the reaction solution into methanol to precipitate polymer, then wash the polymer in a Soxhlet extractor, and finally remove the polymer After drying in a vacuum oven, finally obtain 245mg black polymer P 2 , Yield: 47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com