Preparation method and application of sector hydroxyl zinc fluoride
A sector-shaped zinc hydroxyfluoride technology, which is applied in the field of preparation of sector-shaped zinc hydroxyfluoride catalysts, achieves good stability, is conducive to large-scale promotion, and has simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) After mixing 30ml of water and 5ml of absolute ethanol, under stirring conditions, add 1.75mmol of zinc chloride and 21mmol of sodium hydroxide, stir for 30min,
[0020] 2) Add the mixed solution of step 1) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 140°C for 12 hours, centrifuge and wash for 3 times, and dry at 60°C to obtain zinc oxide product.
[0021] 3) Take 35ml of water, and add the zinc oxide product obtained in step 2) under stirring conditions
[0022] 4) Weigh 1 mmol of zinc oxide product, add 4 mmol of ammonium fluoride, stir for 30 min,
[0023] 5) Add the mixed solution of step 4) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 180°C for 24 hours, centrifuge and wash 3 times, and dry at 60°C to obtain zinc hydroxyfluoride product.
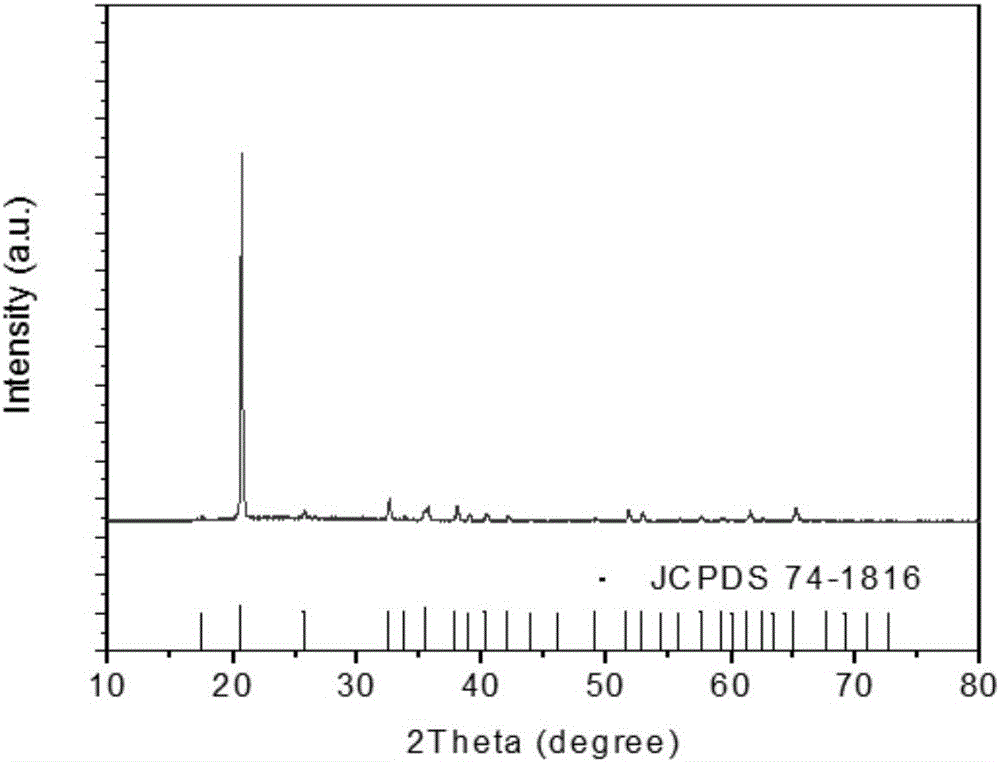
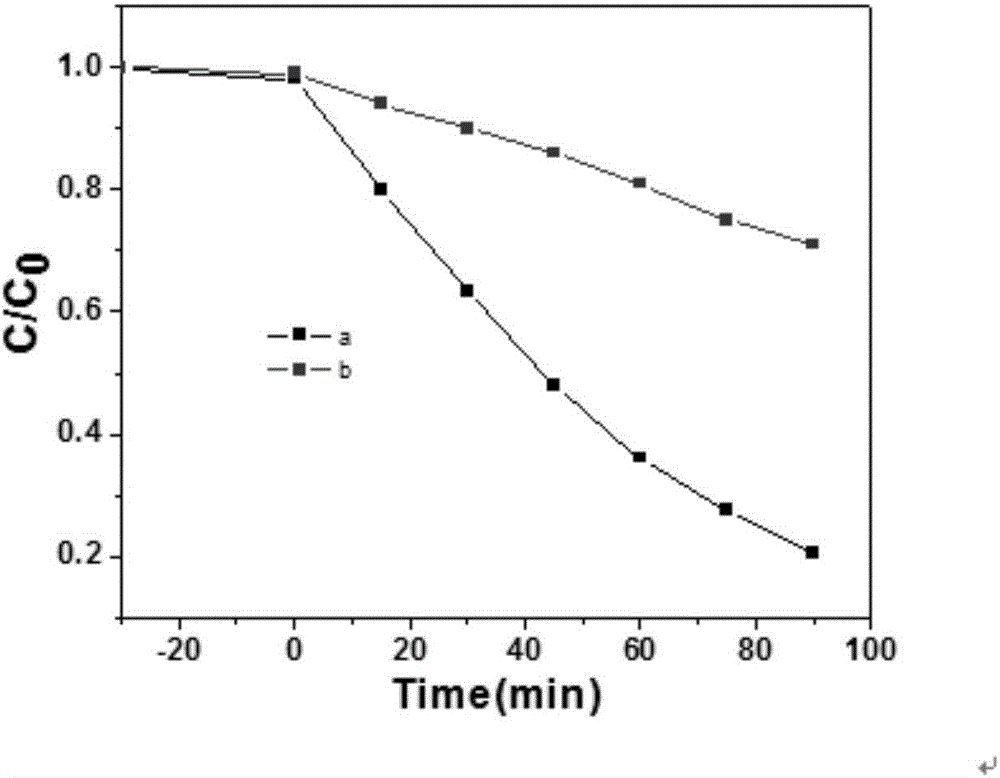
[0024] Depend on figure 1 It can be seen that the zinc hydroxyfluoride catalyst p...
Embodiment 2
[0029] 1) After mixing 30ml of water and 5ml of absolute ethanol, under stirring conditions, add 1.25mmol of zinc chloride and 17mmol of sodium hydroxide, stir for 30min,
[0030] 2) Add the mixed solution of step 1) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 140°C for 12 hours, centrifuge and wash for 3 times, and dry at 60°C to obtain zinc oxide product.
[0031] 3) Take 35ml of water, and add the zinc oxide product obtained in step 2) under stirring conditions
[0032] 4) Weigh 1 mmol of zinc oxide product, add 4 mmol of ammonium fluoride, stir for 30 min,
[0033] 5) Add the mixed solution of step 4) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 180°C for 24 hours, centrifuge and wash 3 times, and dry at 60°C to obtain zinc hydroxyfluoride product.
Embodiment 3
[0035] 1) After mixing 30ml of water and 5ml of absolute ethanol, under stirring conditions, add 2.5mmol of zinc chloride and 24mmol of sodium hydroxide, stir for 30min,
[0036] 2) Add the mixed solution of step 1) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 140°C for 12 hours, centrifuge and wash for 3 times, and dry at 60°C to obtain zinc oxide product.
[0037] 3) Take 35ml of water, and add the zinc oxide product obtained in step 2) under stirring conditions
[0038] 4) Weigh 1 mmol of zinc oxide product, add 4 mmol of ammonium fluoride, stir for 30 min,
[0039] 5) Add the mixed solution of step 4) into an autoclave lined with polytetrafluoroethylene, conduct a hydrothermal reaction at a constant temperature of 180°C for 24 hours, centrifuge and wash 3 times, and dry at 60°C to obtain zinc hydroxyfluoride product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com