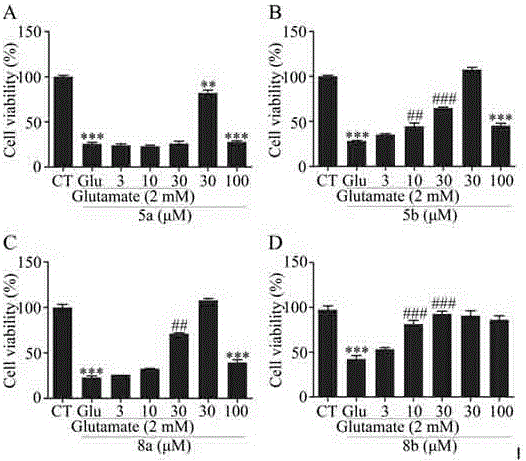
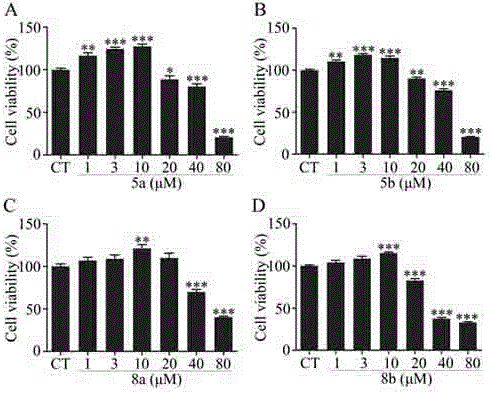
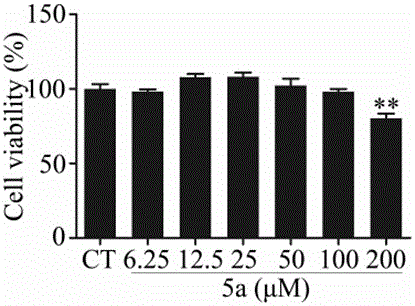
Carbazole-rivastigmine diad and pharmaceutical composition and application thereof
A composition and drug technology, which can be used in drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as cell function changes, oxidative imbalance, and oxidative stress, achieve good neuroprotective effects, and promote proliferation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Compound 2
[0033]
[0034] Material 1 (3.00 g) was dissolved in 100 ml of acetonitrile, and epichlorohydrin (31.00 g) and anhydrous potassium carbonate (2.50 g) were added. The reaction system was reacted at 90°C for 6 hours, 140 ml of water and 200 ml of dichloromethane were added, the layers were extracted and the organic phase was collected. The organic phase was washed once with 100 ml of 0.2 M hydrochloric acid, dried over anhydrous magnesium sulfate, filtered with suction, and concentrated in vacuo to obtain a yellow solid. The initial product was recrystallized from ethyl acetate / petroleum ether to obtain 1.37 g of white solid. Yield: 40%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.48 (d, J = 1.9 Hz, 2H), 7.7-7.64 (m, 2H), 7.64-7.59 (m, 2H), 4.84(dd, J = 15.8, 2.9 Hz, 1H), 4.42 (dd, J = 15.8, 5.8 Hz, 1H), 3.32-3.29 (m, 1H), 2.84-2.62 (m, 1H), 2.55-2.52 (m, 1H).
Embodiment 2
[0035] Example 2: Compound 4a
[0036]
[0037]Mix raw material 3 (0.30 g), dimethylcarbamoyl chloride (0.29 g), and triethylamine (3 g) into a flask, reflux at 95°C overnight, add 50 ml of dichloromethane to dissolve, and concentrate in vacuo at 75°C , add 50 ml of dichloromethane to redissolve, and then wash three times with 0.5 M sodium hydroxide solution. The organic phases were mixed, dried over anhydrous magnesium sulfate, and filtered with suction to obtain a viscous substance. Drying in vacuo yielded 0.29 g of a tan solid. Yield: 59%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.41 (t, J = 7.8 Hz,1H), 6.28 (dd, J = 8.0, 0.5 Hz, 1H), 6.13 (dd, J = 7.6, 0.5 Hz, 1H), 6.08(br, 1H), 2.93 (d, J = 44.0 Hz, 6H).
Embodiment 3
[0038] Example 3: Compound 4b
[0039]
[0040] Mix raw material 3 (0.30 g), N-methylethylcarbamoyl chloride (0.33 g), and triethylamine (3 g) into a flask, reflux at 95°C overnight, add 50ml of dichloromethane to dissolve, and dissolve at 75°C C was concentrated in vacuo, redissolved in 50 ml of dichloromethane, and washed three times with 0.5 M sodium hydroxide solution. The organic phases were mixed, dried over anhydrous magnesium sulfate, and filtered with suction to obtain a viscous substance. Drying in vacuo yielded 0.29 g of a tan solid. Yield: 55%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.41 (t, J =7.8 Hz, 1H), 6.28 (d, J = 7.9 Hz, 1H), 6.13 (d, J = 7.5 Hz, 1H), 6.08 (br,2H), 3.30-3.23 (m, 2H), 2.91 (d, J = 40.2 Hz, 3H), 1.18-1.05 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com