Thioketal linking unit and its synthesis method and application
A thioketal and linking unit technology, applied in the field of thioketal linking unit and its synthesis, can solve the problems affecting the extension efficiency of reversible terminators, DNA strand damage, large molecular traces, etc. Deficiencies and weaknesses, the effect of avoiding the use of acidic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
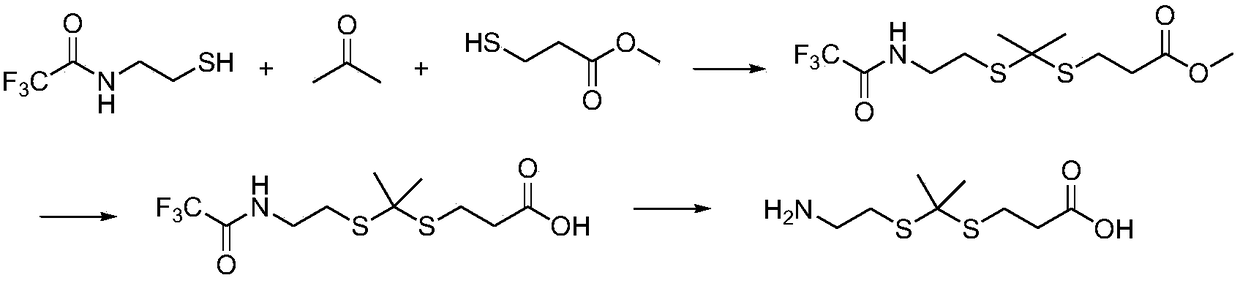
[0065] Embodiment 1, when R 1 = R 2 =Me, R=-COOH, the synthesis of this type of linking unit
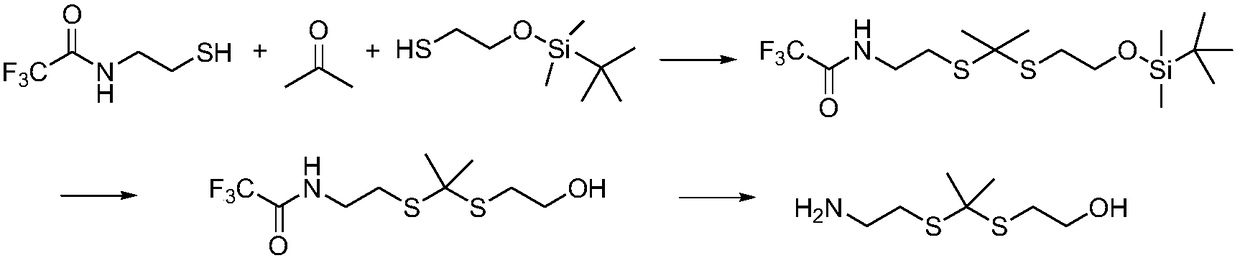
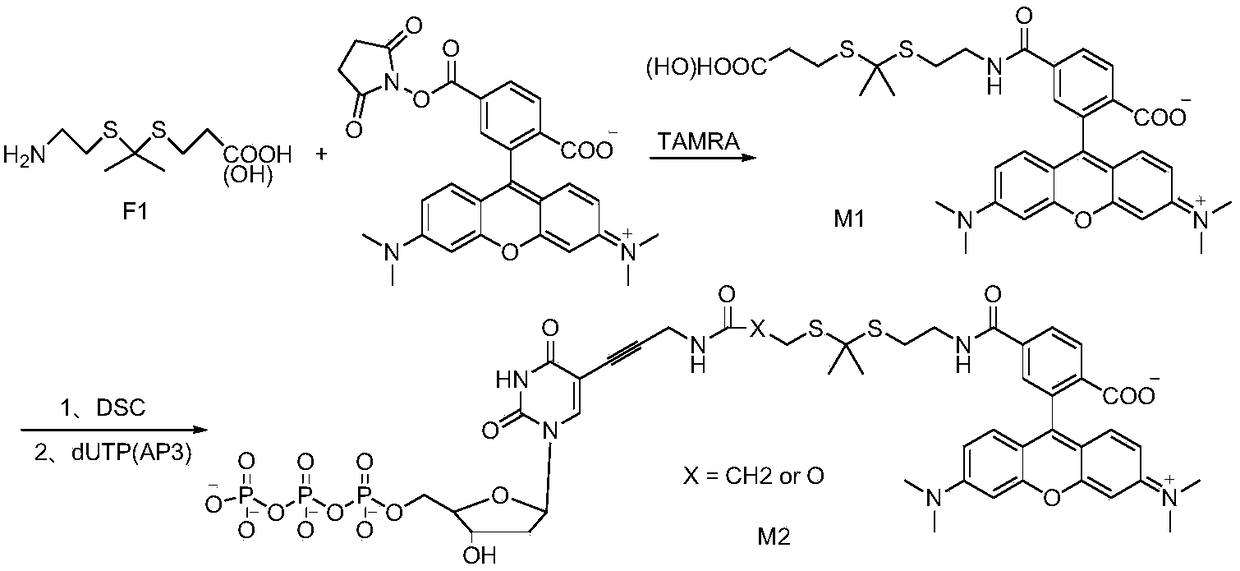
[0066] The schematic diagram of the synthesis of the oxidation-sensitive thioketal linking unit of this example is as follows figure 1 As shown, the specific steps are as follows:
[0067] The first step, the synthesis of compound 5, see the following formula:
[0068]
[0069] Under nitrogen protection, 2-mercaptoethanol hydrochloride (10g, 88mmol) and triethylamine (24mL, 0.17mol) were dissolved in methanol (0.25L), the mixture was cooled to 0°C, and then three Ethyl fluoroacetate (10mL, 84mmol) was added to the reaction mixture, reacted at 0°C for 3h and then continued to react at room temperature for 12h; the solvent was removed with a rotary evaporator, the residue was dissolved in ethyl acetate (0.10L), and water (50mL ) and saturated brine (50 mL) were washed successively, the organic phase was dried over sodium sulfate, and purified by column chromatography to obtai...
Embodiment 2
[0082] Embodiment 2, when R 1 with R 2 Together to form a cyclohexyl group, when R=-COOH, the synthesis of this type of linking unit
[0083] The first step, the synthesis of compound 5: the same as in Example 1.
[0084] The second step, the synthesis of compound 6-1:
[0085]
[0086] Under nitrogen protection, 2-mercaptotrifluoroacetamide 5 (5.7g, 33mmol), methyl 3-mercaptopropionate (4.4g, 37mmol) and cyclohexanone (34mmol) were dissolved in acetonitrile (50mL), and the reaction mixture was cooled to Add boron trifluoride diethyl ether (13mL, 0.11mol) after 0°C, continue the reaction at 0°C for 2h, add 15% sodium carbonate solution (0.30L) to the reaction mixture, extract with ethyl acetate (100mL×3), organic The phase was washed with 5% sodium carbonate solution (0.30 L), dried, and the solvent was spun off, separated and purified by silica gel column to obtain the expected product thioketal 6-1.
[0087] The third step, the synthesis of compound 10-1:
[0088]...
Embodiment 3
[0090] Embodiment 3, when R 1 with R 2 Together to form a cyclopentyl group, when R=-COOH, the synthesis of this type of linking unit
[0091] The first step, the synthesis of compound 5: the same as in Example 1.
[0092] The second step, the synthesis of compound 6-2:
[0093]
[0094] Under nitrogen protection, 2-mercaptotrifluoroacetamide 5 (5.7g, 33mmol), methyl 3-mercaptopropionate (4.4g, 37mmol) and cyclopentanone (34mmol) were dissolved in acetonitrile (50mL), and the reaction mixture was cooled to Add boron trifluoride diethyl ether (13mL, 0.11mol) after 0°C, continue the reaction at 0°C for 2h, add 15% sodium carbonate solution (0.30L) to the reaction mixture, extract with ethyl acetate (100mL×3), organic The phase was washed with 5% sodium carbonate solution (0.30 L), dried, and the solvent was spun off, separated and purified by silica gel column to obtain the expected product thioketal 6-2.
[0095] The third step, the synthesis of compound 10-2:
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com