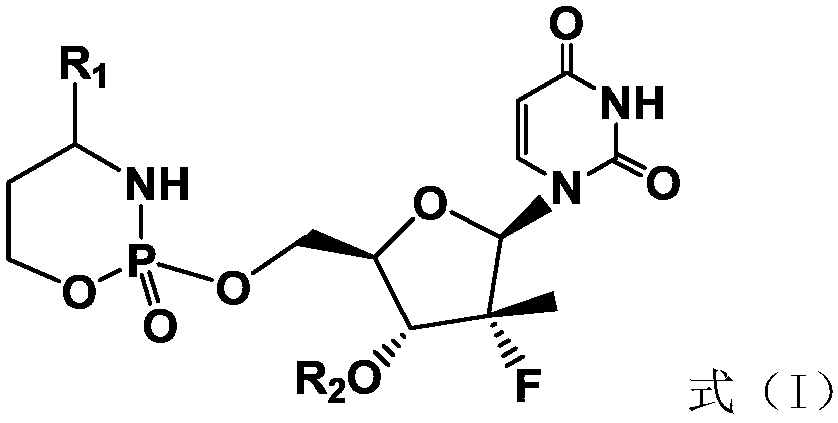
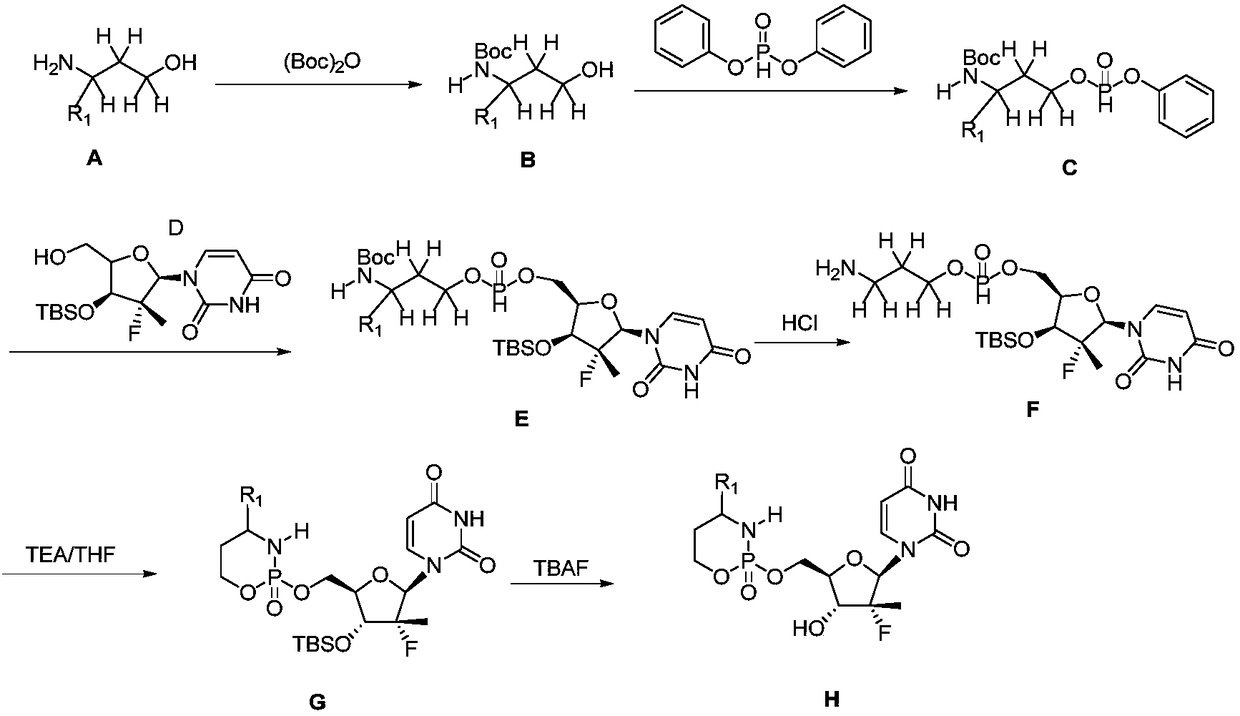
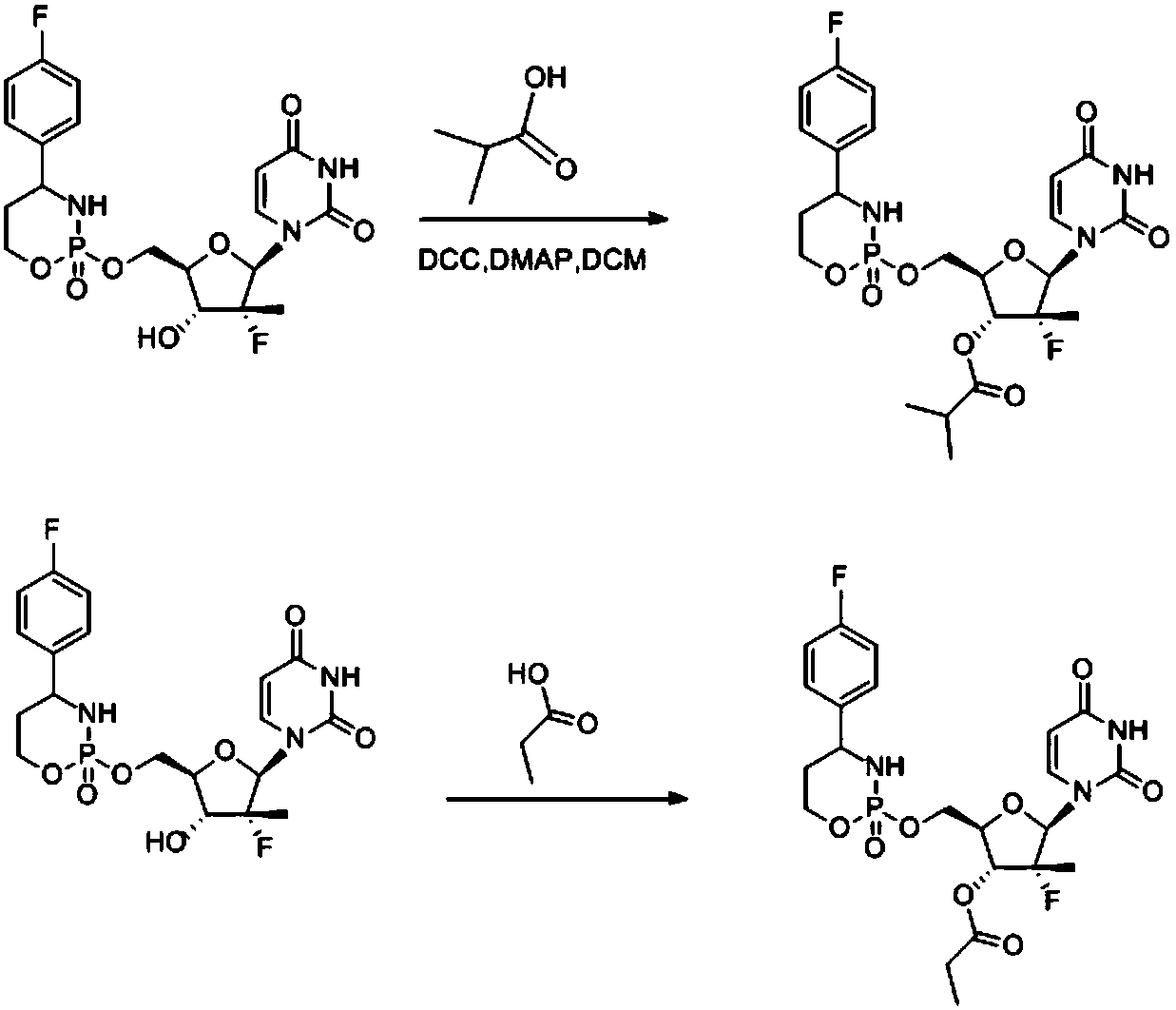
A compound with cyclophosphamide structure and its preparation method
A cyclophosphamide and compound technology, which is applied to the compound having a cyclophosphamide structure and the preparation field thereof, can solve the problems of low bioavailability, poor pharmacokinetic properties, instability in the body and the like, and achieves easy availability of raw materials and safe production process. , the effect of significant industrialization advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 1-((2S,3R,4R,5R)-3-fluoro-4-hydroxyl-3-methyl-5-(((2-oxo-4-phenyl-1,3,2- Preparation of oxazaphosphorin-2-yl)oxy)methyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione (1)
[0034]
[0035] The specific synthesis steps are as follows:
[0036]
[0037] Step 1: 1-((2S,3R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl )-3-fluoro-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione (SF-2)
[0038] At room temperature, to 1-((3R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)- To a DMF solution (200 mL) of diketone (SF-1) (10.4 g, 40 mmol) and imidazole (8.2 g, 120 mmol) was slowly added dropwise a DMF solution (25 mL) of TBSCl (9.0 g, 60 mmol). The reaction system was stirred at 50° C. for 6 h. After TLC showed that the reaction was complete, it was cooled to room temperature, and 150 mL of water was added to quench the reaction, followed by EA extraction (100 mL×4). Combine...
Embodiment 2-7
[0061] Examples 2-7 are obtained through the synthesis route of Example 1, using the above-mentioned intermediates 2b, 3b, 4b, and 5b to obtain the target compound, and the purification is all obtained by reverse-phase preparation. The reverse-phase preparation conditions are as follows:
[0062] 1. Separation solvent: acetonitrile + water
[0063] 2. Separation conditions:
[0064] B% (acetonitrile)
time
Flow rate (mL / min)
5%
5min
20
5%-30%
15min
20
30%-40%
15min
20
95%
5min
20
[0065] The raw materials 2a, 3a, 4a, 5a used are prepared by the following method:
[0066]
[0067] (1) Preparation of xa-2:
[0068] At room temperature, diethyl malonate (1eq) and ammonium acetate (1.5eq) were added sequentially to a solution of the starting material arylaldehyde xa-1 (1eq) in ethanol (about 1.2mol / L). The reaction mixture started to react under the reflux state. After 30 minutes, a large amount of pre...
Embodiment 6
[0074] The target compound 6 of embodiment 6 is prepared through the following reaction with the target compound 5 prepared in embodiment 5:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com