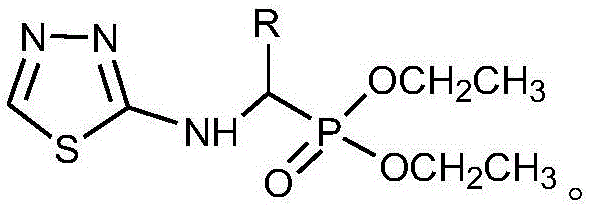
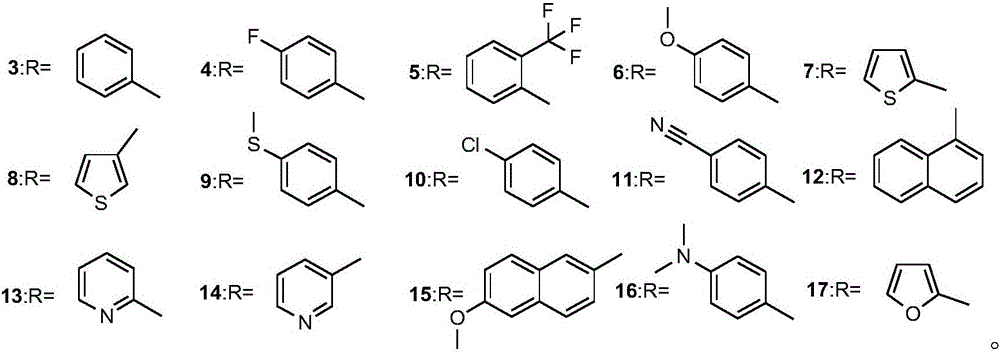
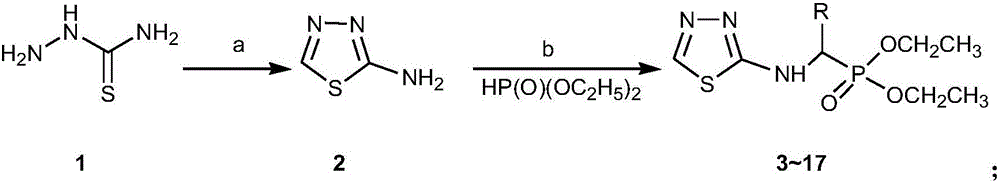Alpha-amino phosphonate compound with 2-amino-1, 3, 4-thiadiazole structure and preparation method and application of alpha-amino phosphonate compound
An aminophosphonate and thiadiazole technology, applied in the field of pesticides, can solve problems such as undiscovered, and achieve the effects of saving time and cost, good economic benefits, and inhibiting growth and proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of O,O'-diethyl-α-(2-amino-1,3,4-thiadiazole)benzyl phosphate:
[0030] Step 1, the preparation of intermediate product
[0031] Weigh 5.00g of thiosemicarbazide, add it to a 50mL three-neck flask with a stirring magnet at one time, add 5.00mL of formic acid, stir and react in an ice bath to form a slurry, and then use a constant pressure dropping funnel to slowly drop 6.00mL concentrated hydrochloric acid. After the dropwise addition, heat in an oil bath at 107°C, and turn on the stirring device, react for 4.5-5.0 hours, follow the reaction by TLC, stop the reaction when the raw material point disappears, and adjust the pH of the system to 8-9 with concentrated ammonia water after cooling at room temperature . Placed in the refrigerator overnight, white crystals precipitated, filtered with suction, washed with ice water three times, and recrystallized with distilled water to obtain 4.96 g of white crystals 1 with a yield of about 90%, m.p.: 198-201°C. Th...
Embodiment 2
[0035] Preparation of O,O'-diethyl-α-(2-amino-1,3,4-thiadiazole)-p-fluorobenzyl phosphate (4):
[0036] Step 1, the preparation of intermediate product
[0037] Add 1.82g (0.02mol) of thiosemicarbazide, 2.54g (0.02mol) of 2-thiophenecarboxylic acid and 20mL of phosphorus oxychloride into the three-necked flask, and slowly raise the temperature to 65°C under magnetic stirring. After 2 hours of reaction, continue to raise the temperature to 106 The reaction was carried out under reflux at ℃ for 4 h, followed by TLC until no raw material was found, and the reaction was stopped. Slowly pour it into ice water while stirring with a glass rod, adjust the pH value to 8-9 with saturated NaOH solution, precipitate out, centrifuge, remove the upper layer, combine the solids, and recrystallize with 75% ethanol to obtain 2.56g of yellow solids 2. Yield: 70%, m.p.: 200-204°C. The structure of the product was determined to be 2-amino-5-thiophene-1,3,4-thiadiazole by IR, NMR and MS analysis...
Embodiment 3
[0041] Preparation of O,O'-diethyl-α-(2-amino-1,3,4-thiadiazole)-o-trifluoromethylbenzyl phosphate:
[0042] Step 1, the preparation of intermediate product
[0043] Add 0.91 g (0.01 mol) of thiosemicarbazide, 2.15 g (0.01 mol) of 2-trifluoromethylbenzoic acid and 20 mL of phosphorus oxychloride into the three-necked flask, and slowly raise the temperature to 65 ° C under magnetic stirring. After 2 hours of reaction, Continue to raise the temperature to 106° C. for reflux reaction for 4 h, TLC traces to the point where there is no raw material, and stop the reaction. Slowly pour it into ice water while stirring with a glass rod, adjust the pH value to 8-9 with saturated NaOH solution, there is precipitation, centrifuge, remove the upper layer, combine the solids, recrystallize with 75% ethanol to obtain 1.99g light green Solid 3, yield 81%, m.p.: 215-221°C. The structure of the product was determined to be 2-amino-5-(trifluoromethyl)phenyl-1,3,4-thiadiazole by IR, NMR and MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com