Treating method for thallium-containing wastewater
A treatment method and wastewater technology, applied in water/sewage treatment, neutralized water/sewage treatment, oxidized water/sewage treatment, etc., can solve the problems of secondary pollution removal rate, high treatment cost, complicated operation, etc., and achieve convenient Subsequent processing, simple operation, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] For the thallium-containing wastewater discharged from a factory, the pH value of the wastewater is 9.0, and the content of thallium is 18.16 mg / L.
[0037] The method of the present invention is used to treat the above-mentioned thallium-containing waste water, which specifically comprises the following steps:
[0038] The first step, adding an oxidant to the thallium-containing wastewater for oxidation treatment, specifically: adding a sodium hypochlorite solution to the thallium-containing wastewater to stir or aerate for 30 minutes, and the stirring rate is 150 rpm.
[0039] The second step is to carry out precipitation treatment on the oxidized thallium-containing waste water. The precipitation treatment is specifically as follows: first, adding sodium sulfide to the oxidized thallium-containing wastewater for precipitation (stirring for 50 minutes), and massaging the sodium sulfide dosage. The molar ratio S (mol): Tl (mol) is 14:1; then add a flocculant for precip...
Embodiment 2- Embodiment 10
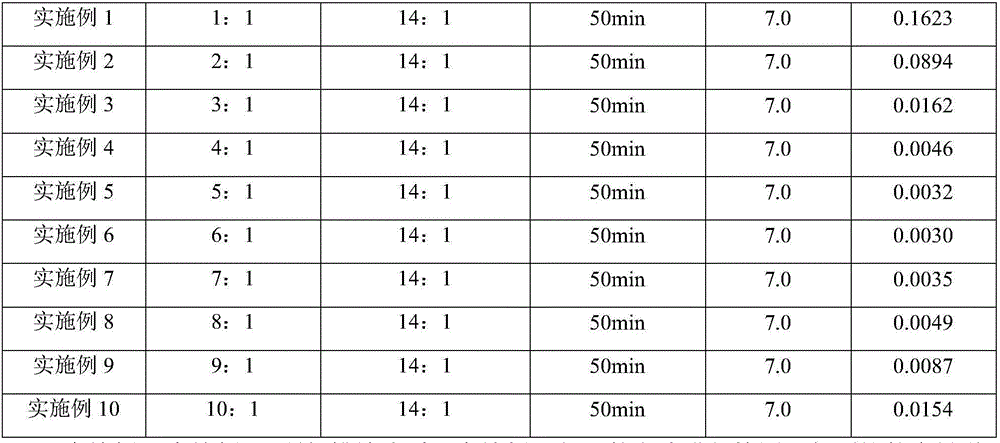
[0043] Example 2-Example 10 differs from Example 1 in Table 1:
[0044] Table 1 The parameter statistics table of embodiment 1-10
[0045]
[0046]
[0047] The discharge liquid obtained in Example 2-Example 10 was detected in the same manner as in Example 1, and the content of thallium obtained is shown in Table 1.
[0048] It can be seen from Table 1 that the amount of oxidant is very important for the treatment of thallium-containing wastewater, and the addition of oxidant can make the Tl in wastewater. + Convert to Tl 3+ , laying the foundation for subsequent precipitation. When the amount of oxidant added is too low (see Example 1-3 for details), T1 + Incomplete conversion will affect the removal effect; when the amount of oxidant added is too high (see Examples 9-10 for details), the unreacted oxidant will react with the subsequent sodium sulfide, affecting the removal effect of Tl. Therefore, the optimal dosage of oxidant Cl(mol):Tl(mol) is 4:1-8:1.
Embodiment 11- Embodiment 15
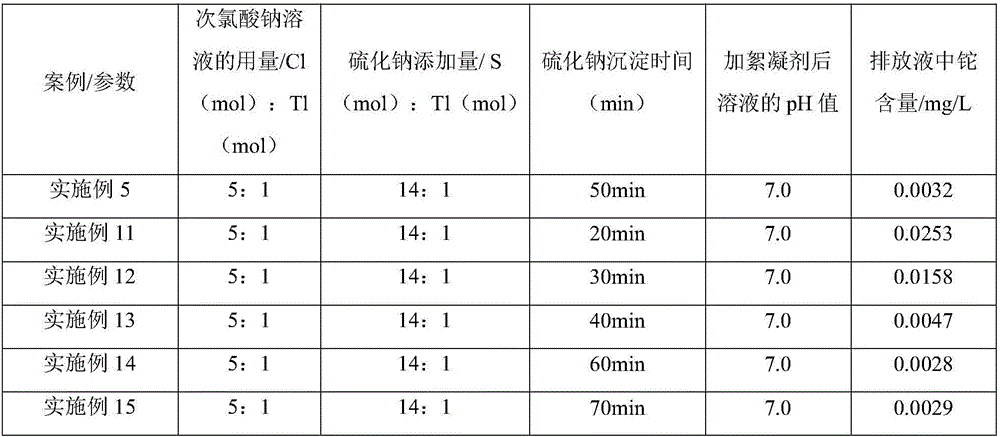
[0050] Example 11-Example 15 differs from Example 5 in Table 2:
[0051] Table 2 Parameter statistics table of embodiment 5 and embodiment 11-15
[0052]
[0053] The effluent obtained in Example 11-Example 15 was tested in the same manner as in Example 1, and the content of thallium obtained is shown in Table 2.
[0054] As can be seen from Table 2, the time of sodium sulfide precipitation treatment is very obvious to the T1 treatment effect in the waste water, and along with the prolongation of time, the precipitation reaction is more and more thorough, and when the precipitation time reaches more than 40min (Example 5, Example 14 -15), the reaction basically tends to be stable, therefore, the optimal reaction time is preferably 40min-60min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com