Acridine marker conjugate and preparation method thereof and chemiluminescence immunoassay kit
A technology of conjugates and markers, which can be used in peptide preparation methods, chemiluminescence/bioluminescence, biological testing, etc., and can solve the problems of decreased activity of acridine-labeled conjugates and affecting the sensitivity of immunoassays, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
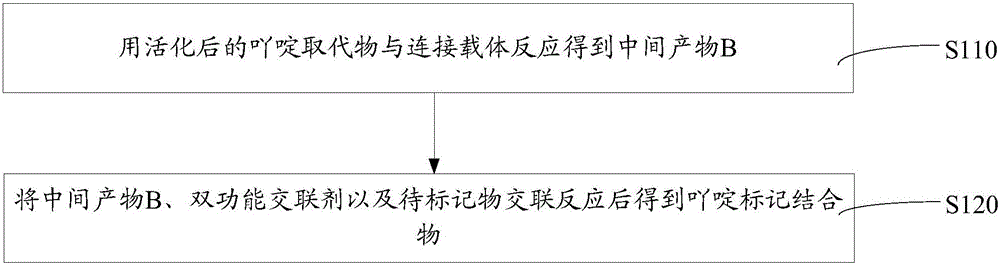
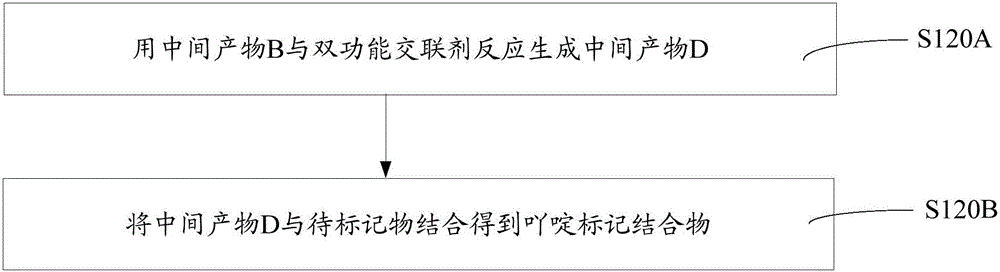
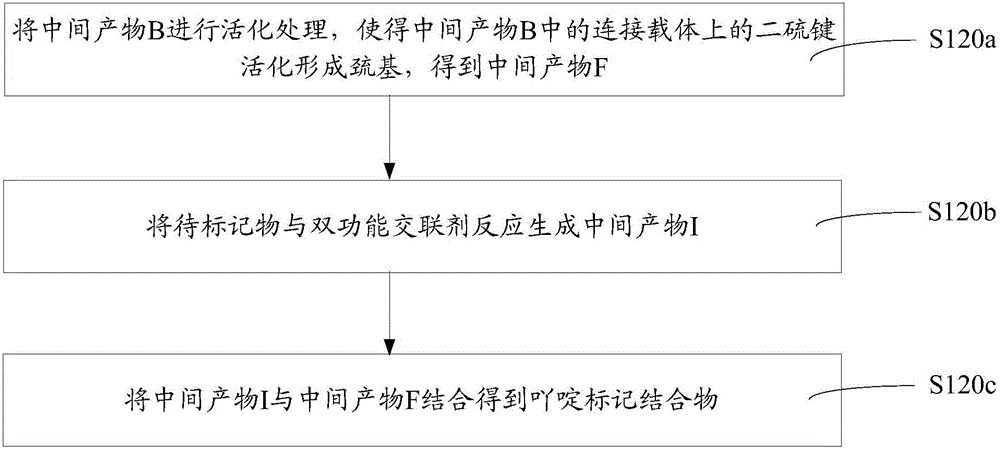
[0050] Such as figure 1 The above-mentioned preparation method of the acridine-labeled conjugate includes the following steps S110-S120.
[0051] S110, using the activated acridine substituent to react with the linking carrier to obtain an intermediate product B.
[0052] Among them, the intermediate product B is an acridine substituent-linking carrier combination, and the linking carrier contains amino and carboxyl groups or the connecting carrier contains amino and disulfide bonds. Further, the connecting carrier can be a connection containing amino, carboxyl and disulfide bonds. The carrier, the acridine substituent reacts with the amino group on the linking carrier to form a -CO-NH- structure so as to connect the acridine substituent to the linking carrier.
[0053] Specifically, the acridine substituent is acridine ester, acridine acid, acridine amide or acridine sulfonamide. Specifically, it can be AE-NHS (10-methyl-acridine-9-N-succinimide ester formate), DMAE-NHS (2'...
Embodiment 1
[0121] Dissolve KLH in 1 mL of 150 mM PBS buffer (pH 7.4) to a final concentration of 20 nmol / L. Add 10 μL of 10 mmol / L acridinium ester dissolved in DMSO solvent. React at 25°C for 1 h, use 5 mL of 7KD molecular weight cut-off desalting column (Thermofish Company) with 150 mM PBS (pH 7.4) buffer as the liquid exchange buffer, and pass through the column for 3 times to remove free acridinium esters and reaction by-products to obtain Acridine ester-KLH solution.
[0122] Dissolve 1 mg of thyroglobulin (manufacturer: biospacific, product number: J19400, 1.5 nmol) in 1 mL of 50 mMMES buffer (pH 4.5), add EDC (final concentration: 5 mmol / L) and EMCH (final concentration: 10 mmol / L), After reacting at 25°C for 30 minutes, use 5mL 7KD molecular weight cut-off desalting column (Thermo fish company) with 150mM PBS (pH7.4) buffer as the replacement buffer, and pass through the column 3 times to remove free EDC, EMCH and By-product, the activated thyroglobulin-EMCH is obtained.
[01...
Embodiment 2
[0126] Dissolve KLH in 1 mL of 150 mM PBS buffer (pH 7.4) to a final concentration of 20 nmol / L, and add 10 μL of 10 mmol / L acridinium ester dissolved in DMSO solvent. React at 25°C for 1 h, use 5mL 7KD molecular weight cut-off desalting column (Thermofish Company) with 50mM MES (pH 4.5) buffer as the replacement buffer, pass through the column for 3 times, remove free acridinium esters and reaction by-products, and obtain Acridine ester-KLH solution.
[0127] EDC (final concentration is 5mmol / L) and EMCH (final concentration is 10mmol / L) are added in the acridinium ester-KLH, after reacting for 30 minutes at 25 ℃, use the desalting column (Thermo fish company) of 5mL 7KD molecular weight cut-off to 150mM PBS (pH 7.4) buffer was used as the replacement buffer, and the column was passed 3 times to remove free EDC, EMCH and by-products to obtain activated acridinium ester-KLH-EMCH;
[0128] Dissolve 1mg of anti-procalcitonin mouse monoclonal antibody antibody (manufacturer: Hyt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com