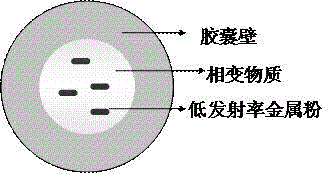
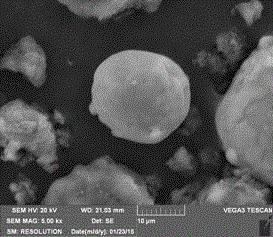
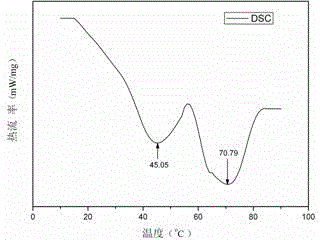
Infrared phase change microcapsule with low emissivity and multiple phase change points and preparation method of infrared phase change microcapsule
A technology of low emissivity and microcapsules, which is applied in the direction of microcapsule preparation, microsphere preparation, chemical instruments and methods, etc., can solve the problems of low emissivity and achieve good anti-oxidation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (3) Dissolve urea in formaldehyde solution in a certain proportion, add polyethylene glycol at the same time, adjust the pH to 7 with triethanolamine, add 5mL of 10% sodium chloride solution, control the reaction temperature to 60°C, and the speed to 400rpm, Obtain modified urea-formaldehyde prepolymer solution component C;
[0023] The mass percentage concentration (the amount of urea, 37% formaldehyde solution, polyethylene glycol, water is calculated by mass ratio) of the substance used in the above reaction process is: urea: 37% formaldehyde solution: polyethylene glycol: water is 1 :1.0-1.5:0.1-0.5:2.0-5.0.
[0024] (4) Take 10 mL of solution B described in step (2), slowly pour it into solution A described in step (1), and stir at room temperature for 30 minutes to obtain mixed emulsion D;
[0025] (5) Slowly pour the mixed emulsion D described in step (4) into the solution C described in step (3), stir vigorously for 5 minutes, adjust the pH to 4-5 with citric a...
Embodiment 2
[0030] (3) Dissolve urea in formaldehyde solution in a certain proportion, add polyethylene glycol at the same time, adjust the pH to 7 with triethanolamine, add 5mL of 10% sodium chloride solution, control the reaction temperature to 60°C, and the speed to 400 rpm , to obtain modified urea-formaldehyde prepolymer solution component C;
[0031] The mass percentage concentration (the amount of urea, 37% formaldehyde solution, polyethylene glycol, water is calculated by mass ratio) of the substance used in the above reaction process is: urea: 37% formaldehyde solution: polyethylene glycol: water is 1 :1.0-1.5:0.1-0.5:2.0-5.0.
[0032] (4) Take 10 mL of solution B described in step (2), slowly pour it into solution A described in step (1), and stir at room temperature for 30 minutes to obtain mixed emulsion D;
[0033] (5) Slowly pour the mixed emulsion D described in step (4) into the solution C described in step (3), stir vigorously for 5 minutes, adjust the pH to 4-5 with cit...
Embodiment 3
[0038] (3) Dissolve urea in formaldehyde solution in a certain proportion, add polyethylene glycol at the same time, adjust the pH to 7 with triethanolamine, add 5mL of 10% sodium chloride solution, control the reaction temperature to 60°C, and the speed to 400rpm, Obtain modified urea-formaldehyde prepolymer solution component C;
[0039] The mass percentage concentration (the amount of urea, 37% formaldehyde solution, polyethylene glycol, water is calculated by mass ratio) of the substance used in the above reaction process is: urea: 37% formaldehyde solution: polyethylene glycol: water is 1 :1.0-1.5:0.1-0.5:2.0-5.0.
[0040] (4) Take 10 mL of solution B described in step (2), slowly pour it into solution A described in step (1), and stir at room temperature for 30 minutes to obtain mixed emulsion D;
[0041] (5) Slowly pour the mixed emulsion D described in step (4) into the solution C described in step (3), stir vigorously for 5 minutes, adjust the pH to 4-5 with citric a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emissivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com