Method for synthesizing 5-chlorin-2-formyl chloride thiofuran by micro-channel reactor
A microchannel reactor, formyl chloride thiophene technology, applied in the direction of organic chemistry, etc., can solve the hidden dangers of material flushing or explosion safety production, inconvenient storage or metering use of acyl chloride reagents, etc., to reduce safety accidents and quality accidents. Possibilities, easy metering, high heat transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
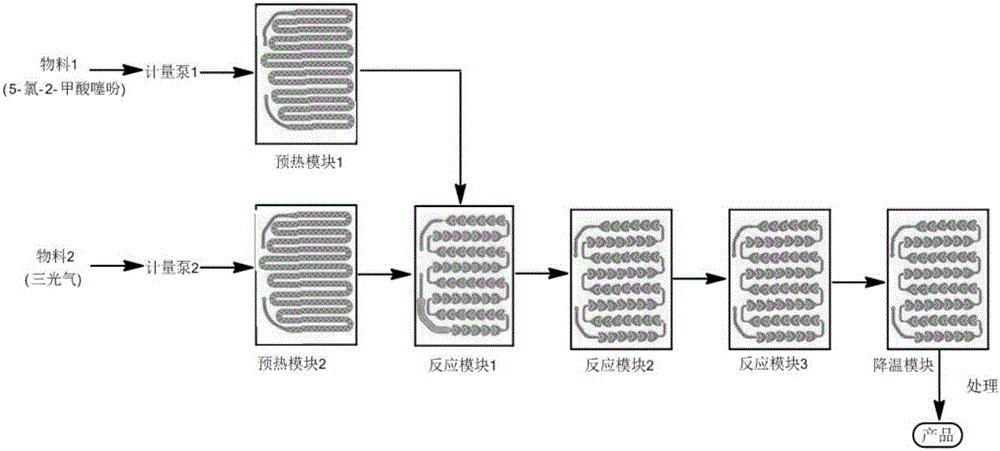
[0036] (1) each module (comprising preheating module, reaction module and cooling module) in the microchannel reactor system that material flows through replaces air with nitrogen;
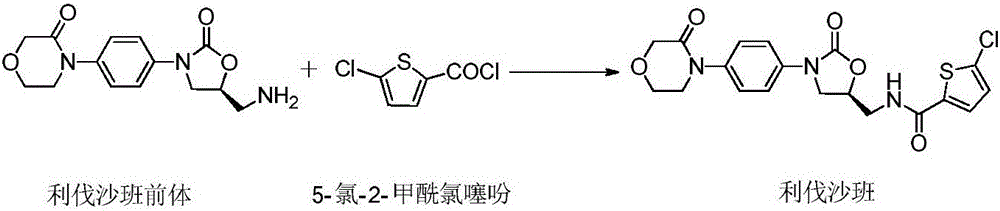
[0037] (2) Add 80.0 g of 5-chloro-2-carboxylic acid thiophene and 40.0 g of DMF into 650 ml of xylene, stir and dissolve as material 1; add 60 g of triphosgene into 400 ml of xylene as material 2;
[0038] (3) The flow rate of material 1 is controlled to be 20ml / min; the flow rate of material 2 is controlled to be 12ml / min; the reaction temperature is 100°C, and the molar ratio of material 1 to material 2 is 1:0.35; the residence time of the reaction is 46s, and the cooling module temperature at 25°C;
[0039] (4) After each strand of material in the reactor reaches a steady state, collect the reaction solution flowing out from the reactor outlet, and feed 30min of material 1 (i.e. 600ml of material 1, 68.5g of 5-chloro-2-formic acid thiophene) Taking the corresponding reaction solution as an exa...
Embodiment 2
[0041] (1) each module (comprising preheating module, reaction module and cooling module) in the microchannel reactor system that material flows through replaces air with nitrogen;
[0042] (2) Add 80 g of 5-chloro-2-carboxylic acid thiophene and 40 g of DMF into 1000 ml of toluene, stir and dissolve as material 1; add 60 g of triphosgene into 600 ml of toluene as material 2;
[0043] (3) The flow rate of control material 1 is 18ml / min; the flow rate of control material 2 is 10ml / min; the reaction temperature is 80°C, and the molar ratio of material 1 and material 2 is 1:0.35; at 25°C;
[0044] (4) After each strand of material in the reactor reaches a steady state, collect the reaction solution flowing out from the reactor outlet, and feed the corresponding reaction of material 1 (i.e. 900ml, 70g of 5-chloro-2-formic acid thiophene) for 50min Liquid as an example, 75.1 g of 5-chloro-2-formylchlorothiophene was obtained after vacuum distillation to separate the solvent toluene,...
Embodiment 3
[0046] (1) each module (comprising preheating module, reaction module and cooling module) in the microchannel reactor system that material flows through replaces air with nitrogen;
[0047] (2) Add 100 g of 5-chloro-2-carboxylic acid thiophene and 50 g of DMF into 615 ml of dioxane, stir and dissolve as material 1; add 75 g of triphosgene into 320 ml of dioxane as material 2 ;
[0048] (3) The flow rate of material 1 is controlled to be 20ml / min; the flow rate of material 2 is controlled to be 10ml / min; the reaction temperature is 50°C, and the molar ratio of material 1 to material 2 is 1:0.4; at 25°C;
[0049] (4) After each strand of material in the reactor reaches a steady state, collect the reaction solution flowing out from the reactor outlet, and pass into the corresponding reaction of material 1 (i.e. 1000ml, 95g of 5-chloro-2-thiophene carboxylate) for 50min Liquid as an example, 101.0 g of 5-chloro-2-formylchlorothiophene was obtained after vacuum distillation and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com