Isoxazole ring derivatives as well as preparation method and application thereof
A derivative and isoxazole technology, which is applied in the field of isoxazole cyclized camptothecin derivatives and their preparation, can solve problems to be optimized and the like, and achieve a combination of maintaining binding capacity, improving biological activity, and reducing the possibility of ring opening. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The preparation method of camptothecin derivative, comprises the steps:
[0058] 3) 10-hydroxy-camptothecin is reacted with hexamethylenetetramine to obtain 9-formylated 10-hydroxy-camptothecin, and then the aldehyde group is reacted with hydroxylamine hydrochloride to generate 9-aldoxime-10-hydroxy-camptothecin Base, under the action of diisopropyl azodicarboxylate and triphenylphosphine, the 9-position aldoxime reacts with the 10-position hydroxyl group to form isoxazole, and generates 9 and 10-position isoxazole camptothecin;
[0059] 4) Esterify the 20-hydroxyl group of 9 and 10-position isoxazole camptothecin with BOC-glycine, remove the BOC protecting group, and acylate the amino terminal of glycine with succinic anhydride to obtain a hydrophilic group at the 20-position The 9,10-position isoxazole camptothecin derivatives, that is, isoxazole cyclized camptothecin derivatives
[0060]
[0061] The reaction steps are as follows:
[0062]
[0063] Applicatio...
Embodiment 1
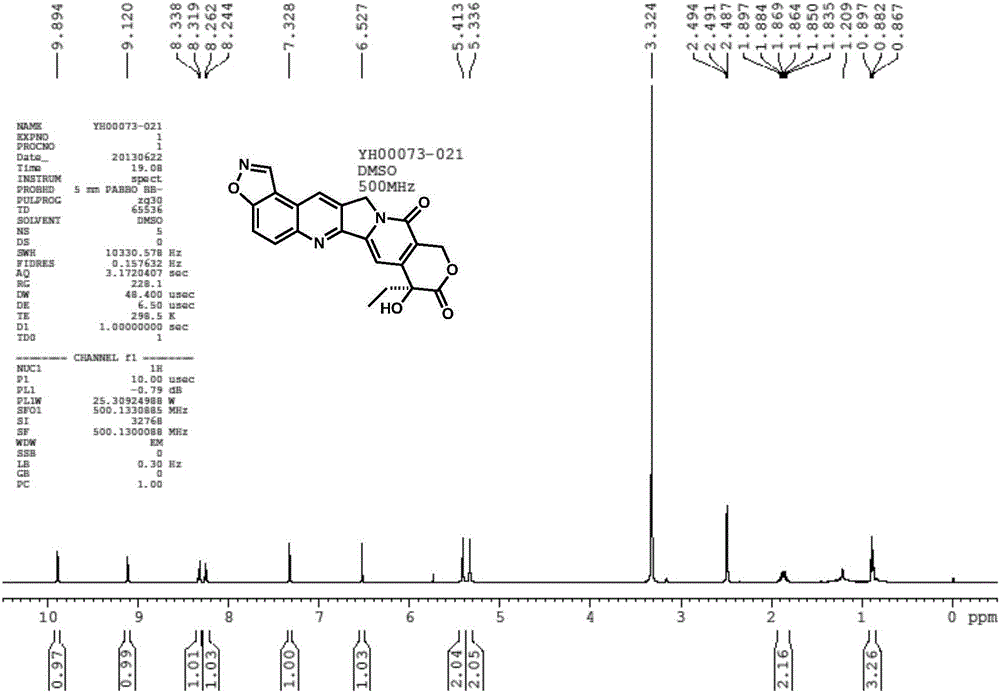
[0080] The isoxazole derivative of camptothecin (quinoline isoxazole compound), the chemical structure formula without hydrophilic group at the 20th position is as follows, the chemical name is: (S)-8-ethyl-8-hydroxy-8H- isoxazolo[4,5-f]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-9,12(11H,14H)-dione
[0081]
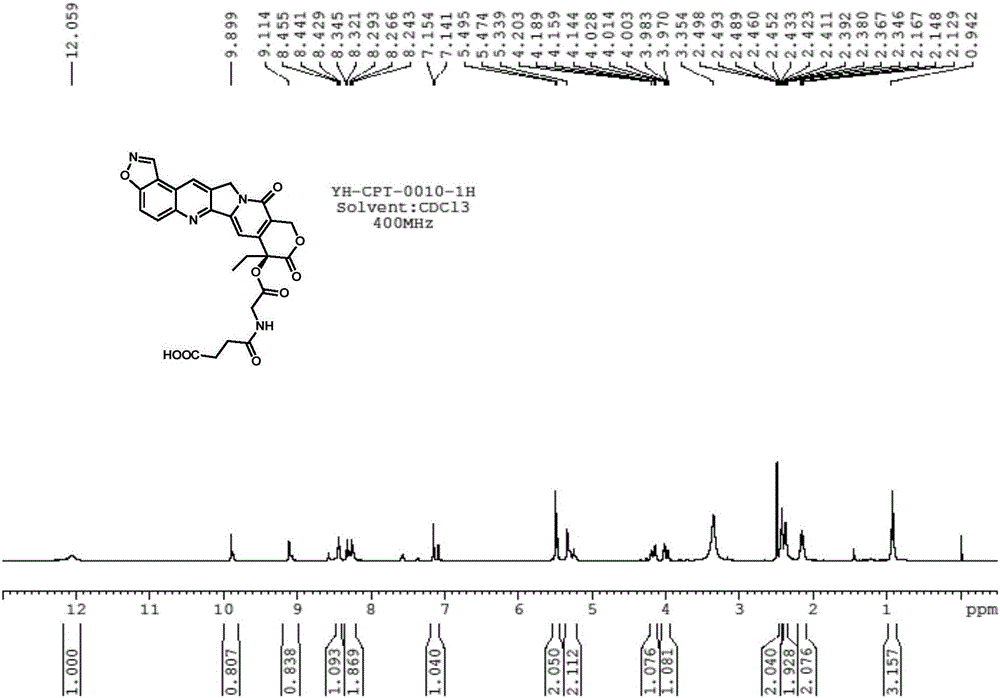
[0082] The chemical name of the isoxazole cyclized camptothecin derivative with a hydrophilic group at the 20th position is: (S)-4-((2-((8-ethyl-9,12-dioxo-9,11, 12,14-tetrahydro-8H-isoxazolo[4,5-f]pyrano[3',4':6,7]indolizino[1,2-b]quinolin-8-yl)oxy)-2-oxoethyl)amino )-4-oxobutanoic acid, its chemical structure is as follows:
[0083]
[0084] The preparation method of above-mentioned camptothecin derivatives comprises the following steps, and its synthetic route is as follows:
[0085]
[0086]
[0087] Concrete synthetic steps are as follows:
[0088] 1) A solution of 10-hydroxycamptothecin (compound 1, 100 mg, 0.27 mmol) and hexamethylenetetramine (HMTA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com