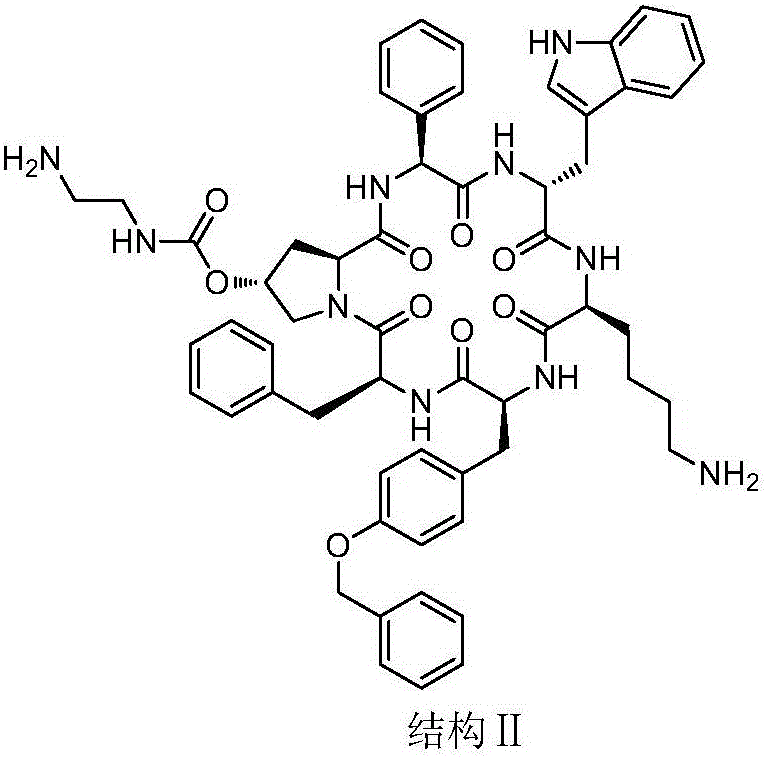
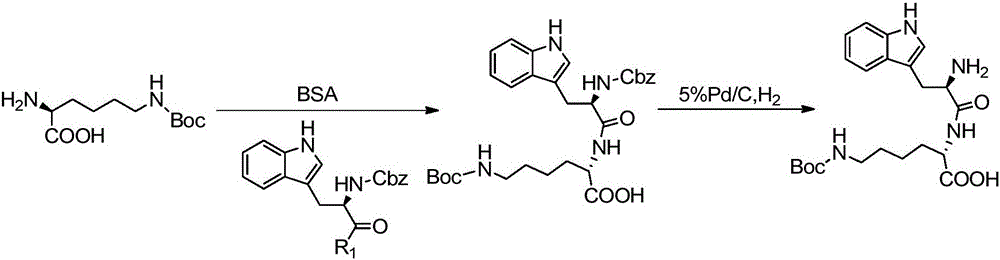
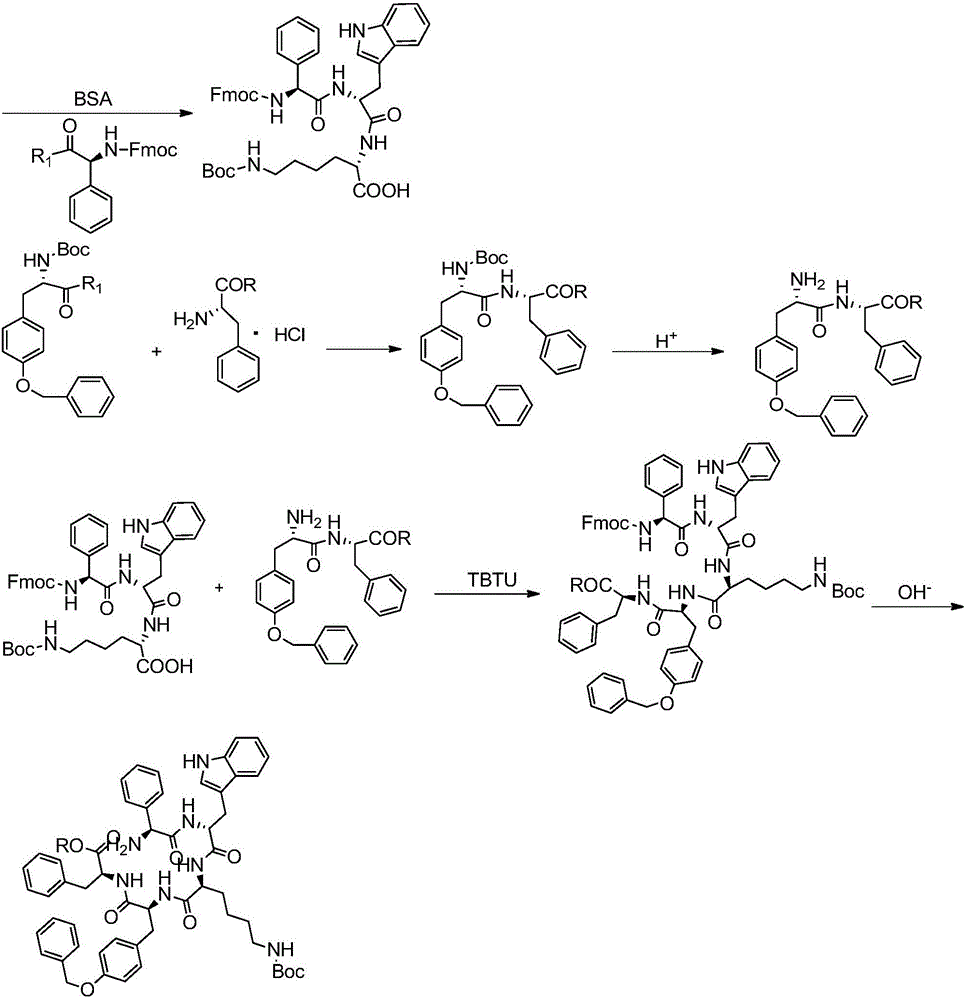
Synthesis and application of pasireotide pentapeptide intermediate
A technology of pasireotide pentapeptide and retinide pentapeptide is applied in the application field of preparing pasireotide, can solve the problems of difficult detection of reaction, high cost, unsuitability for large-scale production and the like, and achieves rapid reaction and high efficiency. Complete, easy-to-monitor, and easy-to-scale results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0060] Preparation Example 1 N-Benzyloxycarbonyl-D-tryptophan-(N-hydroxyl)succinimide ester (a)
[0061]
[0062] N-Benzyloxycarbonyl-D-tryptophan (7.0 g, 20.7 mmol) and N-hydroxysuccinimide (2.4 g, 20.7 mmol) were added to anhydrous THF (80 ml), and stirred at room temperature. The temperature was controlled at about 5°C, and a THF (20ml) solution of DCC (4.3g, 20.9mmol) was slowly added dropwise. Stir at room temperature for 24h, filter several times, and concentrate the filtrate to dryness under reduced pressure. The obtained yellow oil was recrystallized from isopropanol to obtain 7.6 g of off-white solid with a yield of 84.6%. 1 HNMR (400MHz, DMSO) δ10.94 (s, 1H), 8.14 (d, J = 8.2Hz, 1H), 7.56 (d, J = 7.9Hz, 1H), 7.31 (ddd, J = 24.4, 17.1, 8.5 Hz,5H),7.09(t,J=7.2Hz,1H),7.00(t,J=7.2Hz,1H),4.99(q,J=12.6Hz,2H),4.72-4.59(m,1H), 3.35(dd,J=14.6,4.7Hz,1H),3.17(dd,J=14.6,9.9Hz,1H),2.80(d,J=31.4Hz,4H)
preparation example 2
[0063] Preparation Example 2 N-(9-fluorenylmethoxycarbonyl)phenylglycine-(N-hydroxyl)succinimide ester (b)
[0064]
[0065] N-(9-fluorenylmethoxycarbonyl)phenylglycine (10 g, 26.8 mmol) and N-hydroxysuccinimide (3.1 g, 26.8 mmol) were added to anhydrous THF (100 ml), and stirred at room temperature. Control the temperature at about 5°C, slowly drop into a solution of DCC (5.6g, 27.1mmol) in THF (20ml), and stir at room temperature for 24h. After several times of filtration, the filtrate was concentrated to dryness under reduced pressure, and ethyl acetate was added for recrystallization to obtain 11.2 g of white solid with a yield of 89.2%. 1 H NMR (400MHz, CDCl3) δ7.76(d, J=7.3Hz, 2H), 7.64-7.53(m, 2H), 7.40(t, J=7.4Hz, 2H), 7.31(t, J=7.4Hz ,2H),5.79(s,1H),4.54(m,J=8.1Hz,1H),4.45-4.40(m,1H),4.39-4.23(m,1H),2.81(s,4H)
preparation example 3
[0066] Preparation Example 3 N-tert-butoxycarbonyl-O-benzyl-tyrosine-(N-hydroxy)succinimide ester (c)
[0067]
[0068] Add N-tert-butoxycarbonyl-O-benzyl-tyrosine (7.4g, 19.9mmol) and N-hydroxysuccinimide (2.3g, 19.9mmol) to anhydrous THF (80ml), stir at room temperature . Control the temperature at about 5°C, slowly drop into a solution of DCC (4.1g, 20.1mmol) in THF (20ml), and stir at room temperature for 5h. After several times of filtration, the filtrate was concentrated to dryness under reduced pressure, and ethyl acetate was added for recrystallization to obtain 8.5 g of white solid with a yield of 91.0%. 1 H NMR (400MHz, CDCl3) δ7.47-7.29 (m, 4H), 7.20 (d, J = 8.1Hz, 2H), 6.93 (d, J = 8.1Hz, 2H), 5.04 (s, 2H), 4.89 (d,J=12.6Hz,1H),3.31-3.06(m,2H),2.85(s,4H),1.42(s,9H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com