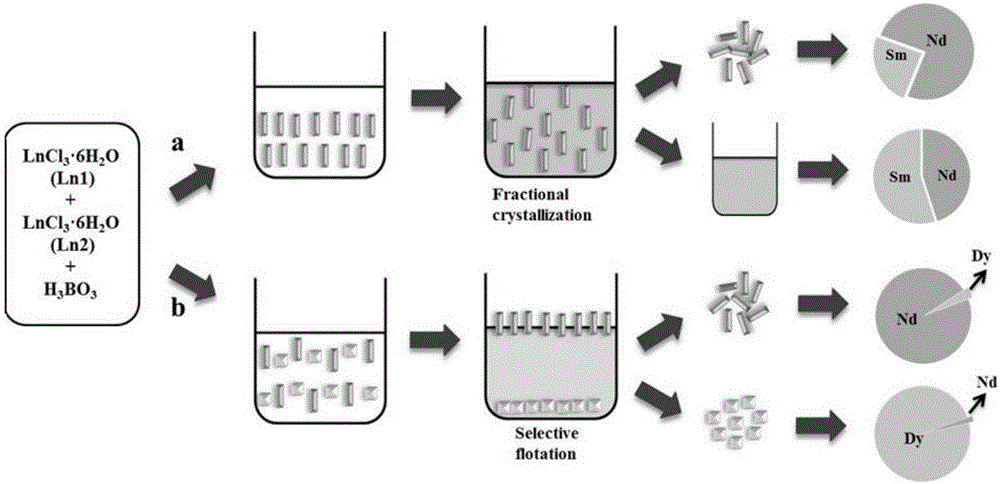
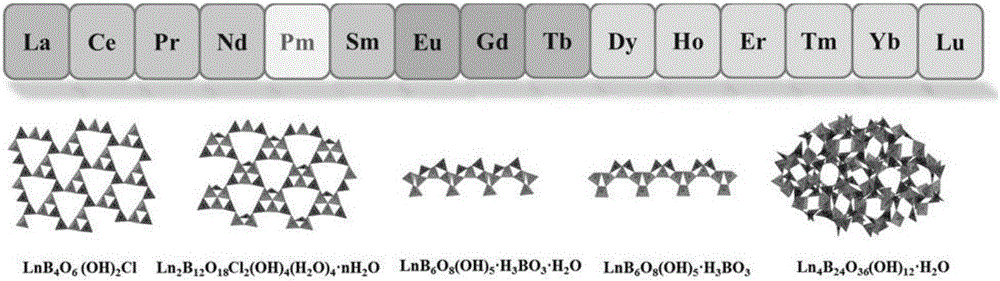
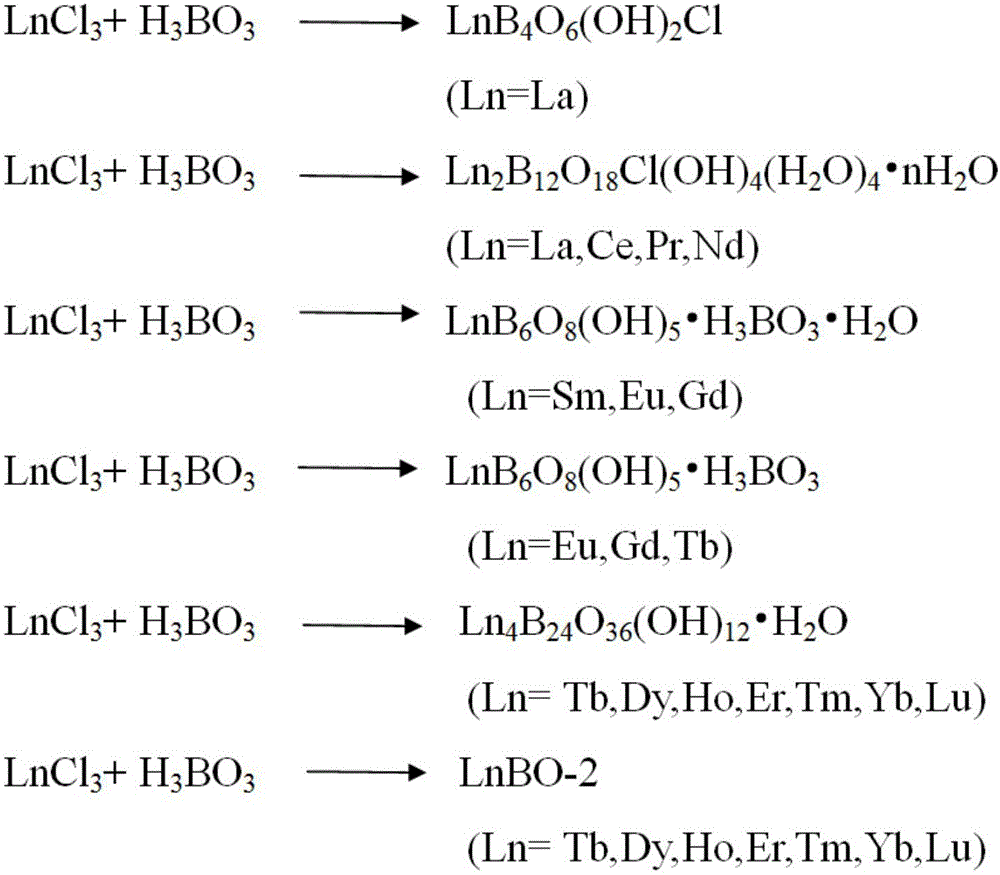Method for separating lanthanide series elements
A lanthanide, thermal method, applied in chemical instruments and methods, crystal growth, liquid solvent solutions at room temperature, etc. Efficient crystallization separation, avoiding multiple separations, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment one: the separation of neodymium samarium (NdSm)
[0041] 0.3mmol NdCl 3 ·6H 2 O, 0.3 mmol SmCl 3 ·6H 2 O and 4.5 mmol H 3 BO 3 Put it in a 10ml polytetrafluoroethylene reaction kettle, add 0.1ml deionized water, seal it, heat up to 190°C, heat for 3 days, then cool to room temperature at a rate of 3-4oC / h, wash the product with a large amount of boiling water, and wash the lotion Collect them into a 100ml volumetric flask and make up to volume, and the resulting crystalline product is washed with ethanol and dried at room temperature.
[0042] The resulting crystals were characterized by a powder diffractometer, such as Figure 4Shown, prove that this doping reaction only generates the crystal of a kind of structure type, and this crystal has identical structure with NdBOCl crystal; Crystal is first dissolved with concentrated nitric acid, then diluted to low acidity, with inductively coupled plasma mass spectrometer ( ICP-Ms) measure the concentratio...
Embodiment 2
[0043] Embodiment two: the separation of neodymium dysprosium (NdDy)
[0044] 0.3mmolNdCl 3 ·6H 2 O, 0.3mmol DyCl 3 ·6H 2 O and 4.5 mmol H 3 BO 3 Put it in a 10ml polytetrafluoroethylene reaction kettle at a ratio of 1:1:15, add 0.1ml of deionized water, seal it, heat it up to 200°C, heat it for 3 days, and then cool it to room temperature at a rate of 3-4oC / h. After washing with a large amount of boiling water, the obtained crystal product was washed with ethanol and dried at room temperature, and the washing liquid was collected into a 100ml volumetric flask to constant volume.
[0045] The resulting crystals were characterized by a powder diffractometer, such as Image 6 As shown, it proves that the doping reaction only generates crystals of one structure type, and the crystals have the same structure as NdBOCl crystals; the crystals are first dissolved with concentrated nitric acid, then diluted to low acidity, and analyzed by an inductively coupled plasma mass spect...
Embodiment 3
[0046] Embodiment three: the separation of neodymium dysprosium (NdDy)
[0047] 0.3mmolNdCl 3 ·6H 2 O, 0.3mmol DyCl 3 ·6H 2 O and 6.0 mmol H 3 BO 3 Put it in a 10ml polytetrafluoroethylene reaction kettle at a ratio of 1:1:20, add 0.1ml of deionized water, seal it, heat it up to 210°C, heat it for 7 days, and then cool it to room temperature at a rate of 3-4oC / h. Wash with a large amount of boiling water, wash the product crystals with ethanol and dry at room temperature, and collect the washing liquid into a 100mL volumetric flask to constant volume.
[0048] The resulting product was characterized by a powder diffractometer, such as Figure 8 As shown, it was proved that the product contained three crystal types (NdBOCl, DyBO-1 and DyBO-2) with different structures.
[0049] Put the product in bromoform, stir well, let it stand for a period of time, and selectively suspend or precipitate in the bromoform solution due to the different densities of crystals of different...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com