Preparation method of polyvinylidene fluoride-hexafluoropropylene diaphragm
A technology of polyvinylidene fluoride and hexafluoropropylene, applied in electrolytic coating, electrophoretic plating, coating, etc., can solve the problems of difficult control of diaphragm pore distribution, uneven current distribution, and uneven pore distribution, etc., to improve industrial Production efficiency, production efficiency improvement, uniform pore distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
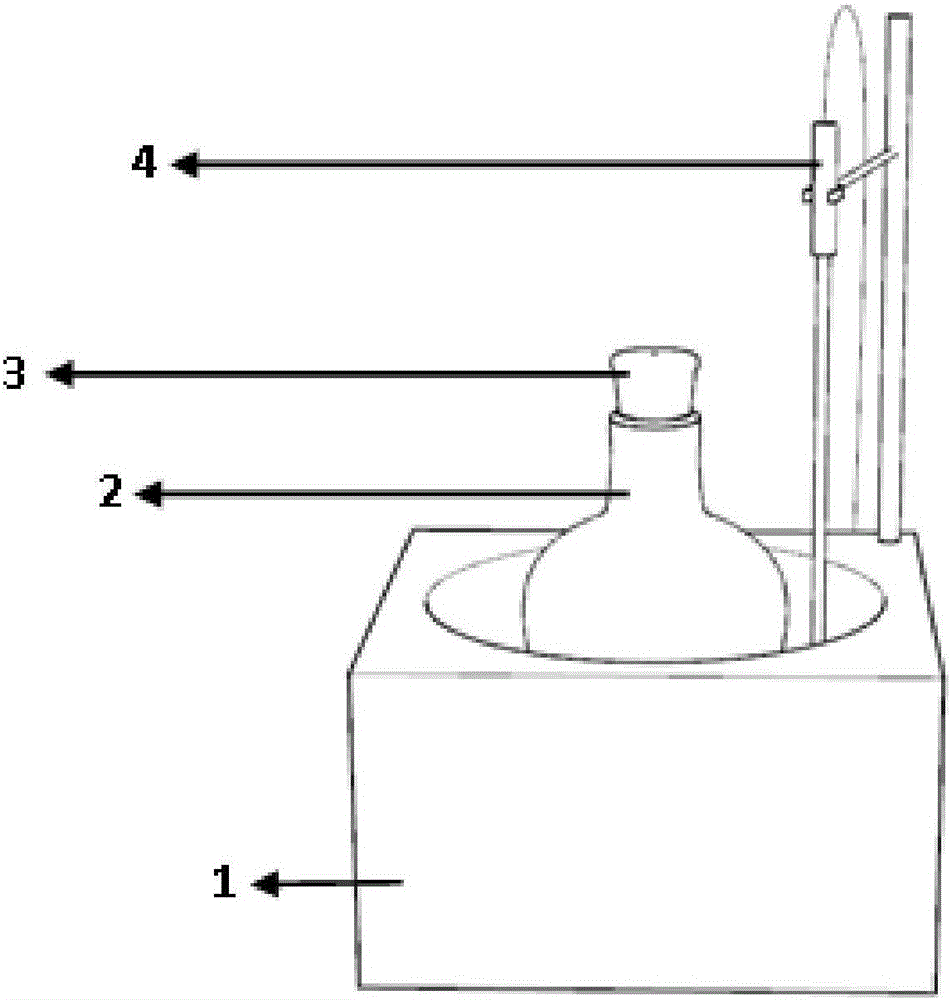
[0031] In this example, the figure 1 and figure 2 The shown setup is used for electrolyte preparation and performing electrophoresis.
[0032] S1: Raw materials: weigh polyvinylidene fluoride-hexafluoropropylene powder and solid iodine at a mass ratio of 40:1
[0033] Solvent: appropriate amount of acetone;
[0034] S2: Add polyvinylidene fluoride-hexafluoropropylene powder and acetone into the round bottom flask 2, place the round bottom flask 2 in the magnetic stirring water bath 1, when the temperature of the water bath is about 30°C, the stirring time is about 8- 10min, until the polyvinylidene fluoride-hexafluoropropylene powder is uniformly dispersed in acetone;
[0035] S3: Add weighed solid iodine into the round bottom flask 2, when the temperature of the water bath is about 15°C, stir for about 15-20min until the solid iodine dissolves in acetone to prepare an electrolyte;
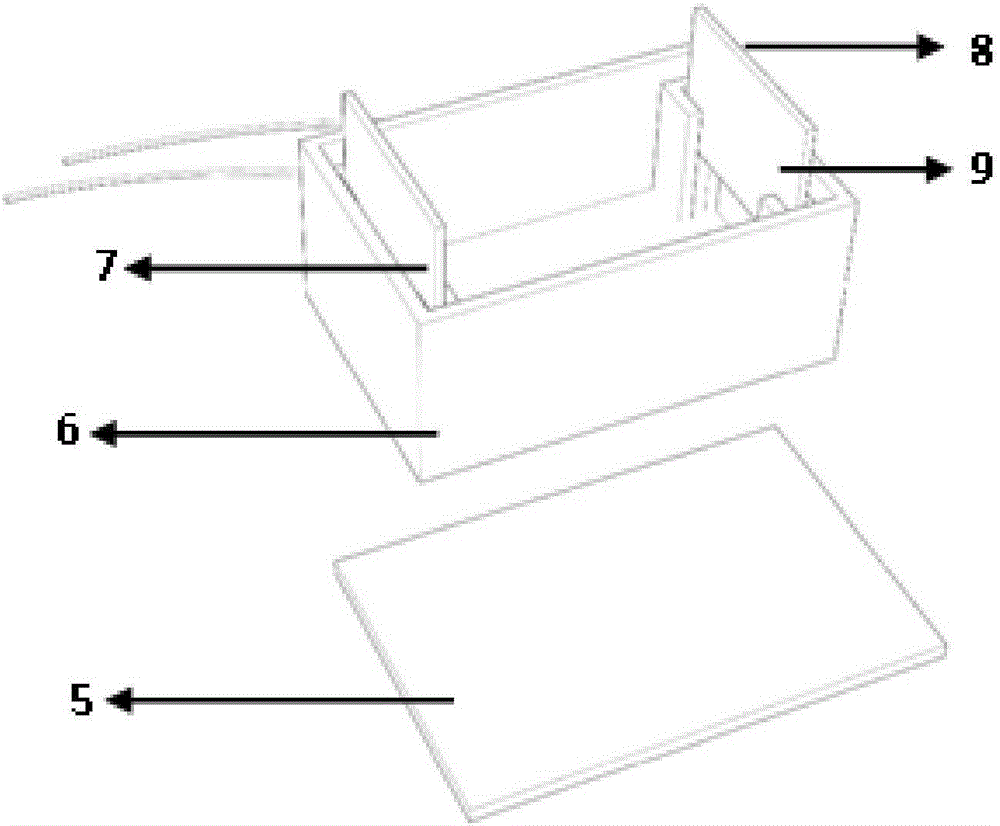
[0036]S4: Pour the electrolyte solution into the electrophoresis tank 6, insert the graph...
Embodiment 2
[0038] In this example, the figure 1 and figure 2 The shown setup is used for electrolyte preparation and performing electrophoresis.
[0039] S1: Raw materials: weigh polyvinylidene fluoride-hexafluoropropylene powder and solid iodine at a mass ratio of 45:1
[0040] Solvent: appropriate amount of acetone;
[0041] S2: Add polyvinylidene fluoride-hexafluoropropylene powder and acetone into the round bottom flask 2, place the round bottom flask 2 in the magnetic stirring water bath 1, when the temperature of the water bath is about 40°C, the stirring time is about 6- 8min until the polyvinylidene fluoride-hexafluoropropylene powder is uniformly dispersed in acetone;
[0042] S3: Add weighed solid iodine into the round bottom flask 2, when the temperature of the water bath is about 20°C, stir for about 12-17min until the solid iodine dissolves in acetone to prepare an electrolyte;
[0043] S4: Pour the electrolyte solution into the electrophoresis tank 6, insert the graphi...
Embodiment 3
[0045] In this example, the figure 1 and figure 2 The shown setup is used for electrolyte preparation and performing electrophoresis.
[0046] S1: Raw materials: weigh polyvinylidene fluoride-hexafluoropropylene powder and solid iodine at a mass ratio of 55:1
[0047] Solvent: appropriate amount of acetone;
[0048] S2: Add polyvinylidene fluoride-hexafluoropropylene powder and acetone into the round bottom flask 2, place the round bottom flask 2 in the magnetic stirring water bath 1, when the temperature of the water bath is about 50°C, the stirring time is about 4- 6min until the polyvinylidene fluoride-hexafluoropropylene powder is uniformly dispersed in acetone;
[0049] S3: Add weighed solid iodine into the round bottom flask 2, when the temperature of the water bath is about 25°C, stir for about 9-14min until the solid iodine dissolves in acetone to prepare an electrolyte;
[0050] S4: Pour the electrolyte solution into the electrophoresis tank 6, insert the graphit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com