Methylene bridging 1,8-naphthyridine ligand and copper (I) complex, preparing method and application
A methylene bridge and complex technology, applied in the chemical field, can solve the problems of high cost, toxic and harmful heavy metal residues, etc., and achieve the effect of shortening the reaction process and simplifying raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
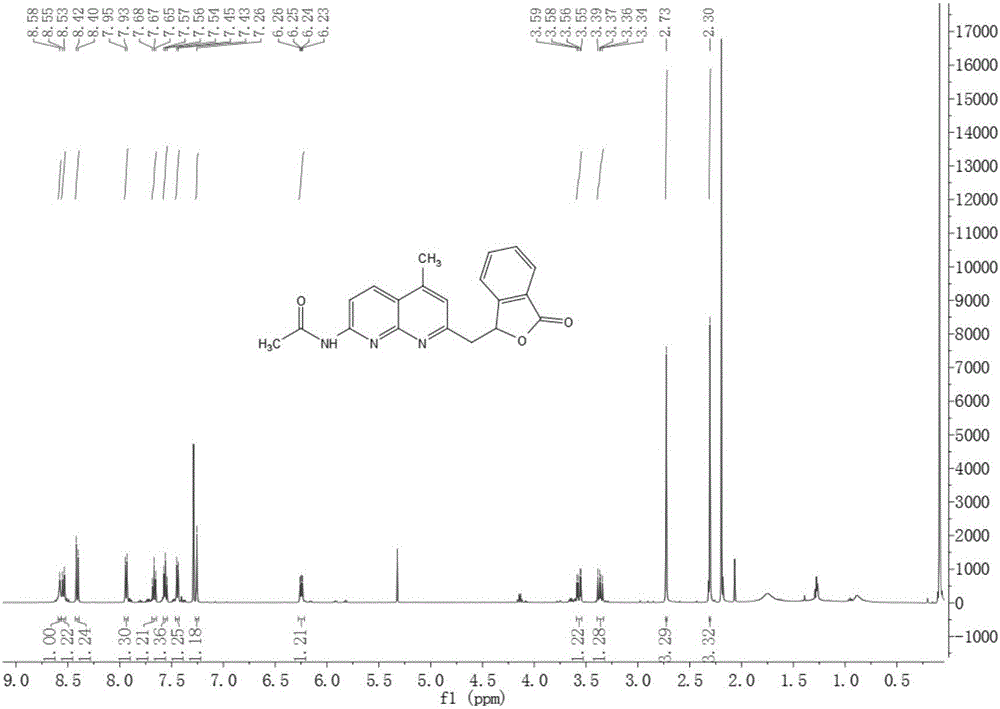
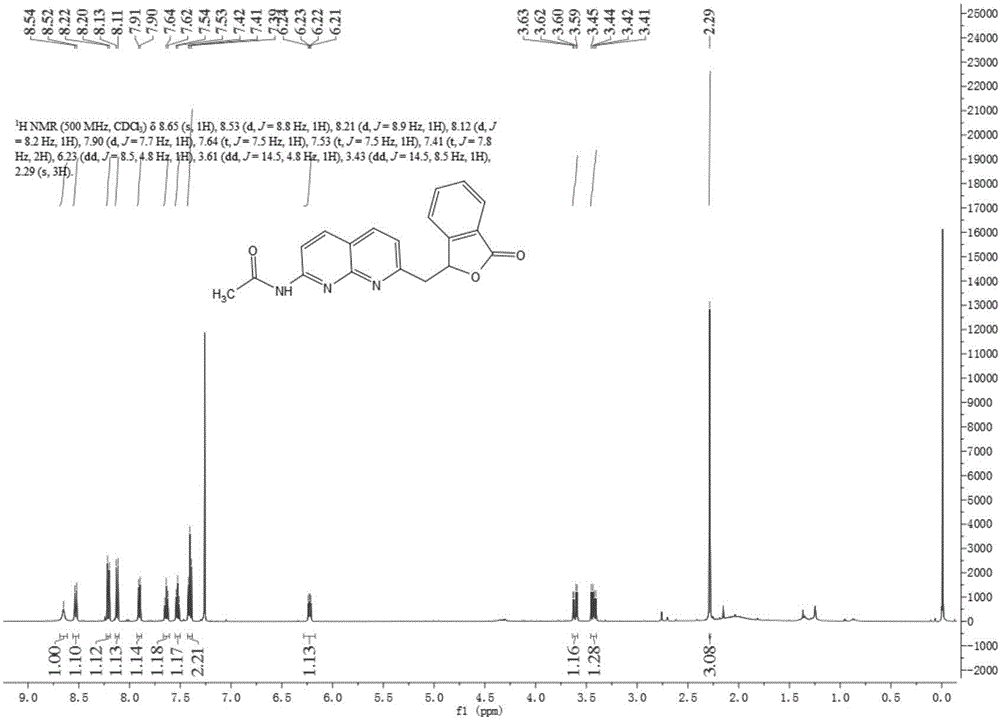
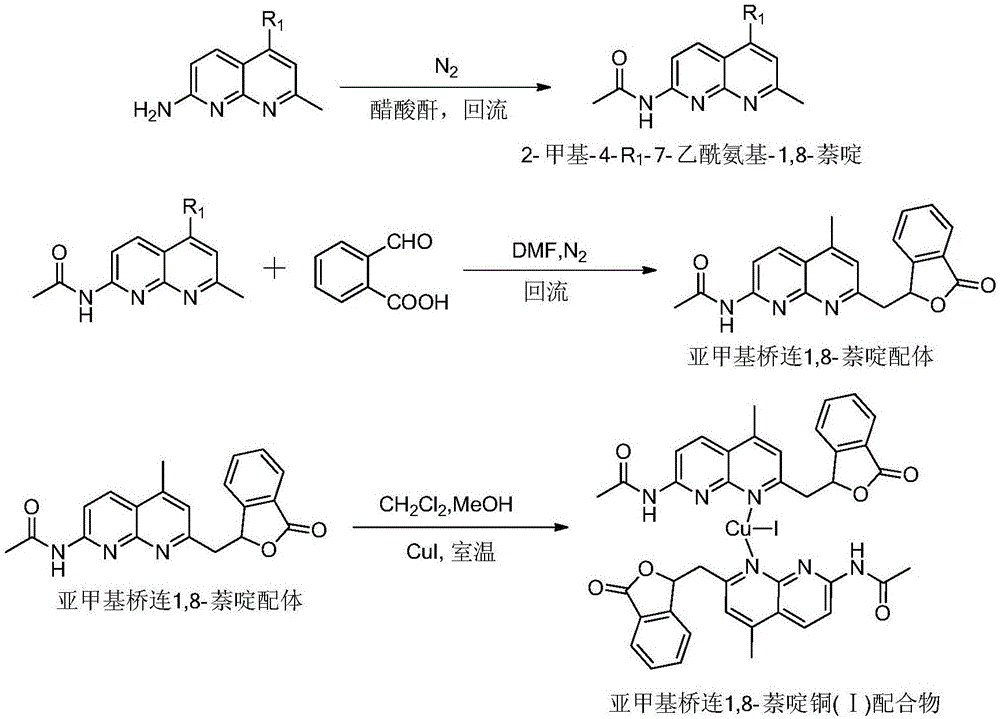
[0042] 1. Synthesis of methylene bridged 1,8-naphthyridine ligand and copper (I) complex:
[0043] 1) Synthesis of 2-methyl-4-R 1 -7-Acetamido-1,8-naphthyridine:
[0044] Add 7-amino-2,4-dimethyl-1,8-naphthyridine or 7-amino-2-methyl-1,8-naphthyridine (4.0 g, 23.1 mmol) into a 100 mL round bottom flask, add 15mL of acetic anhydride, the mixture was refluxed for 2h under the protection of nitrogen, and the reaction liquid was cooled to precipitate yellow crystals, which were filtered to obtain 7-acetamido-2,4-methyl-1,8-naphthyridine or 7-acetamido-2-methyl -1,8-naphthyridine product is directly used in the following reaction;
[0045] 2) No catalyst SP 3 Synthesis of methylene bridged 1,8-naphthyridine ligands by C—H activation:
[0046] Dissolve 0.35g (2.33mmol) o-aldehyde benzoic acid in 30mL N,N-dimethylformamide, raise the temperature to 150°C under nitrogen protection, and then add 0.50g (2.32mmol) 7-acetamido-2,4 -Dimethyl-1,8-naphthyridine and continue to reflux for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com