PH-triggering-to-release multilevel targeting polymer micelle in tumor cells and preparation method of multilevel targeting polymer micelle
A technology of tumor cells and polymer glue, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as chemotherapy failure, achieve saturated drug resistance mechanisms, improve anti-tumor efficacy, and overcome multi-drug resistance of tumors Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
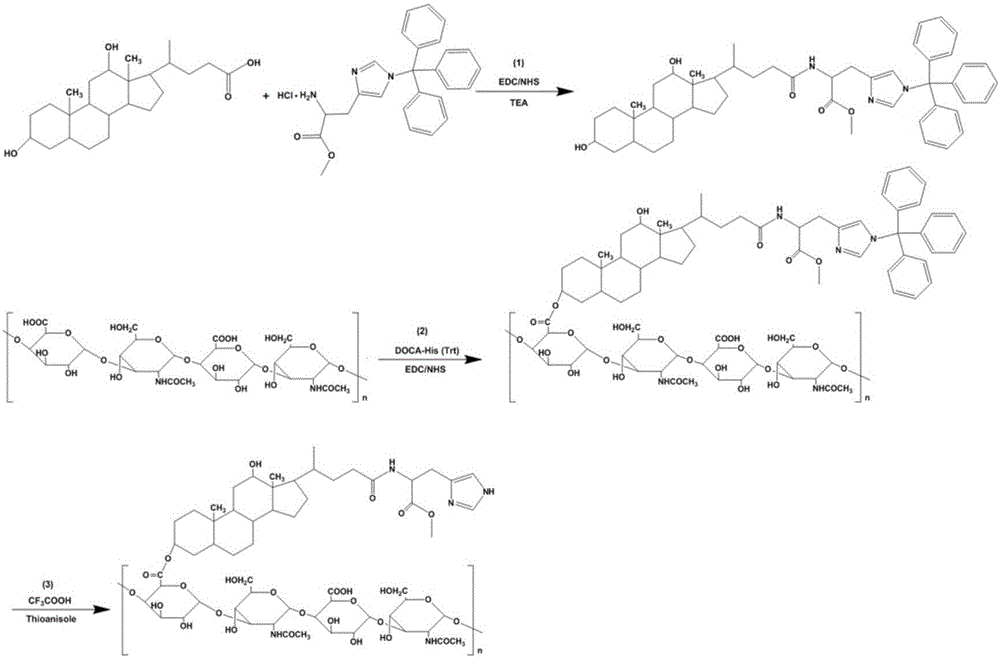
[0046] Example 1: Synthesis of HA-DOCA-His polymer
[0047] 0.5 g DOCA was dissolved in 5 mL N,N-dimethylformamide, 0.29 g EDC and 0.18 g NHS were added, and stirred at room temperature for 2 h. 0.37 g of trityl histidine methyl ester hydrochloride (H-His (Trt)-OMe HCl) was dissolved in 20 mL of DMF, and 50 µL of triethylamine was added, and slowly added dropwise to the DOCA mixture, the mixture Stir in a water bath at 35 °C for 24 h. NaHCO was added to the reaction mixture 3 solution (pH 9-10), the precipitate was obtained by suction filtration, and dried under reduced pressure to obtain DOCA-His (Trt).
[0048] Dissolve 0.1 g HA in 5 mL anhydrous formamide, heat to dissolve at 50°C, and cool to room temperature. Add 96 mg EDC and 58 mg NHS, and stir in an ice bath for 2 h. 0.4 g DOCA-His(Trt) was dissolved in 5 mL of anhydrous DMF, slowly added dropwise to the HA mixture, stirred in a water bath at 50 °C for 6 h, and then stirred at room temperature for 24 h. The reacti...
example 2
[0050] Example 2: Synthesis of HA-DOCA-His polymer
[0051] 0.5 g DOCA was dissolved in 5 mL N,N-dimethylformamide, 0.29 g EDC and 0.18 g NHS were added, and stirred at room temperature for 24 h. 0.37 g of trityl histidine methyl ester hydrochloride (H-His (Trt)-OMe HCl) was dissolved in 20 mL of DMF, slowly added dropwise to the DOCA mixture, and the mixture was stirred in a water bath at 35 °C for 24 h. The reaction mixture was spun to remove the organic solvent, and separated by a column to obtain DOCA-His (Trt).
[0052] 0.1 g HA was dissolved in 5 mL of anhydrous formamide, heated to dissolve at 50 °C, and cooled to room temperature. Add 96 mg EDC and 58 mg NHS, and stir in an ice bath for 2 h. 0.4 g DOCA-His(Trt) was dissolved in 5 mL of anhydrous DMF, slowly added dropwise to the HA mixture, stirred in a water bath at 50 °C for 6 h, and then stirred at room temperature for 24 h. The reaction mixture was dialyzed in distilled water for 2-3 days (dialysis bag molecula...
example 3
[0054] Example 3: Synthesis of HA-DOCA-His polymer
[0055] 0.5 g DOCA was dissolved in 5 mL N,N-dimethylformamide, 0.29 g EDC and 0.18 g NHS were added, and stirred at room temperature for 24 h. 0.37 g of trityl histidine methyl ester (H-His(Trt)-OH) was dissolved in 20 mL of DMF, slowly added dropwise to the DOCA mixture, and the mixture was stirred in a water bath at 35 °C for 24 h. The reaction mixture was rotary evaporated to remove the organic solvent, and separated by column to obtain DOCA-His(Trt).
[0056] 0.1 g HA was dissolved in 5 mL anhydrous formamide, heated to dissolve at 60 °C, and cooled to room temperature. Add 192 mg EDC and 116 mg NHS, and magnetically stir in ice bath for 5 h. 0.8 g DOCA-His (Trt) was dissolved in 5 mL of anhydrous DMF, slowly added dropwise to the HA mixture, and stirred at room temperature for 48 h. Ethanol was added to the reaction mixture to precipitate and remove impurities to obtain HA-DOCA-His (Trt) white powder.
[0057] Disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com