Mitoxantrone estrogen targeting PEG-modified liposome and applications thereof
An anthraquinone estrogen, mitoxantrone technology, applied in the directions of liposome delivery, antitumor drugs, drug combination, etc., can solve the problems of short metabolism time, low drug concentration, expensive raw materials, etc., and achieve high curative effect and distribution. Uniform and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
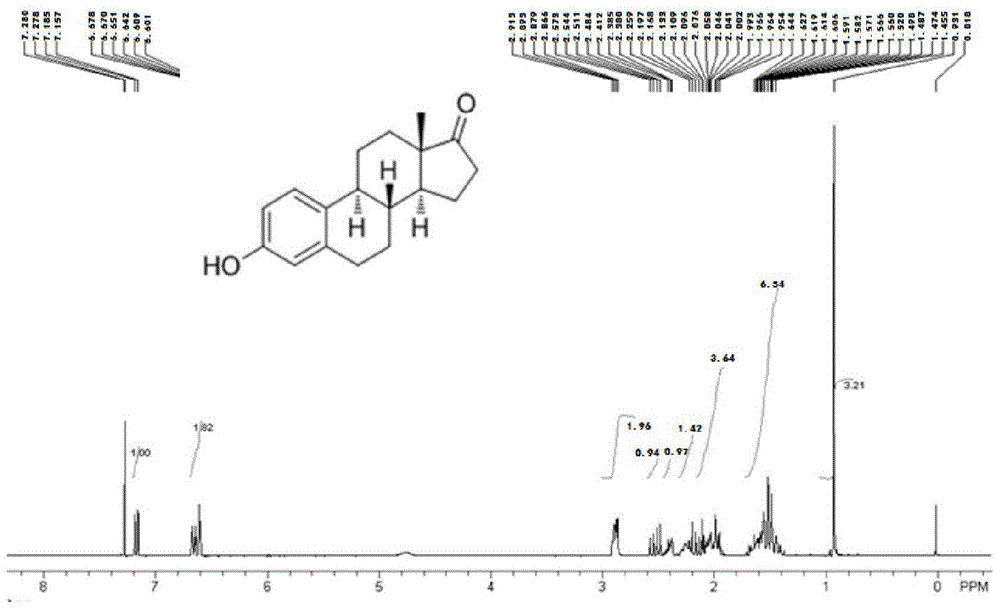
[0061] Embodiment 1: Synthesis of estrone-pegylated distearoylphosphatidylethanolamine
[0062] 1. Preparation of activated estrone ester: Weigh 108.00 mg of estrone, 50.00 mg of succinic anhydride, 48.80 mg of 4-dimethylaminopyridine and 40.48 mg of triethylamine and dissolve them in dioxane. Stir overnight at room temperature. Dioxane was evaporated to dryness under vacuum, the residue was dissolved in dichloromethane and filtered. The filtrate was further concentrated, precipitated with ether, and dried to obtain the activated ester of estrone.
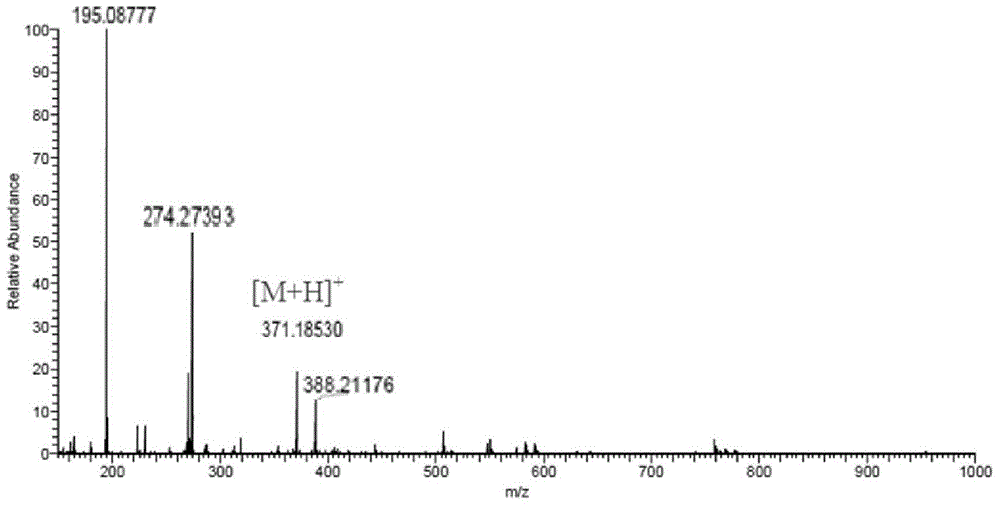
[0063] 2. Synthesis of estrone-pegylated distearoylphosphatidylethanolamine: 92.50 mg of estrone activated ester obtained above was dissolved in methylene chloride and 6.75 mg of hydroxybenzotriazole. Under ice cooling, 51.55 mg of dicyclohexylcarbodiimide and 137.50 mg of aminopegylated distearoylphosphatidylethanolamine were added. The resulting solution was stirred continuously for 18 hours until a precipitate formed. The pr...
Embodiment 2
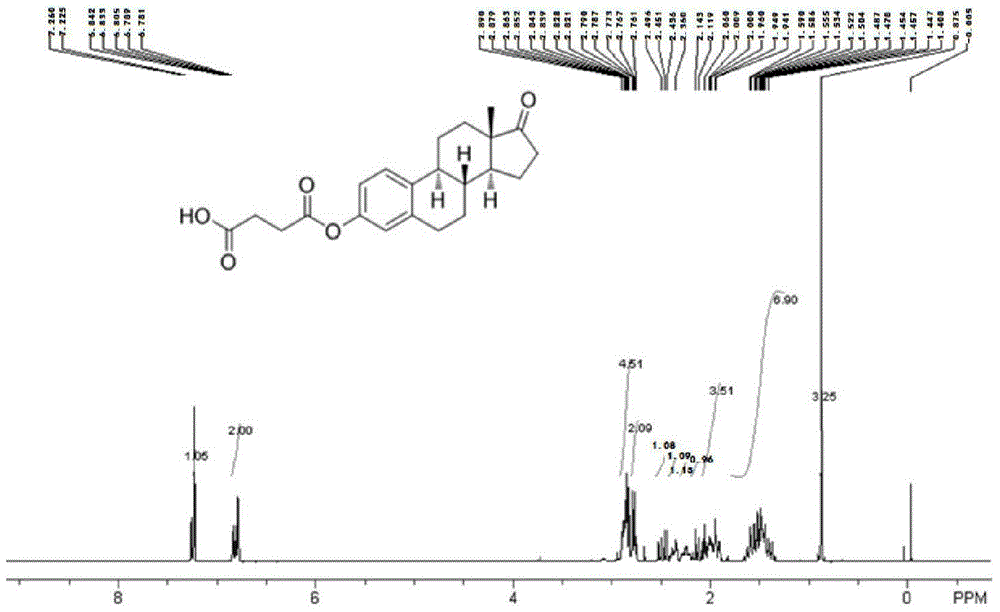
[0064] Example 2: Synthesis of fulvestrant-pegylated dipalmitoylphosphatidylethanolamine
[0065] 1. Preparation of fulvestrant activated ester: Weigh 1122.50 mg of fulvestrant, 370.00 mg of succinic anhydride, 561.00 mg of triethylamine and 122.00 mg of 4-dimethylaminopyridine, dissolve them in 4 ml of dioxane, place React in a 10ml round-bottomed flask at 25°C for 48h under magnetic stirring, evaporate to dryness under reduced pressure, add 10mL of water in an ice-water bath and adjust the pH to 1 with 5mol / L HCl solution, after standing still, a precipitate precipitates, and filter under reduced pressure to obtain the product Fulvestre Group activated esters.
[0066] 2. Synthesis of fulvestrant-pegylated dipalmitoylphosphatidylethanolamine: dissolve 95.40 mg of the above fulvestrant activated ester in 4 ml of dichloromethane, add 18.24 mg of hydroxybenzotriazole and dicyclohexyl carbon Diimine 21.90 mg, reacted at room temperature for 1 hour, then added aminopegylated dip...
Embodiment 3
[0067] Example 3: Mitoxantrone estrogen-targeted PEG-modified liposome 1
[0068] Using the ammonium sulfate gradient method: take 200 mg of soybean lecithin, 50 mg of cholesterol, and 100 mg of polyethylene glycol-distearoylphosphatidylethanolamine and dissolve them in 30 ml of methanol, remove the organic solvent by rotary evaporation, and then add 10 ml of 250 mM ammonium sulfate aqueous solution to 60 ° C water for 0.5h. After sonicating for 5 min with a probe sonicator, it was dialyzed 4 times in 5% glucose solution. Add 20 mg of mitoxantrone and incubate for 1 hour, then dialyze, then add 10 mg of estrone-polyethylene glycol-distearoylphosphatidylethanolamine for incubation, and use an extruder to extrude through a 0.22 μm polycarbonate film for 10 times to obtain rice Toxantrone estrogen-targeted PEG-modified liposome formulation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com