Brain targeted medicine lipidosome preparation as well as preparation method and application thereof
A pharmaceutical preparation and brain-targeting technology, which is applied in liposome delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of not being able to penetrate the blood-brain barrier and not having brain-targeting properties, and achieve low toxicity and good stability , the effect of promoting intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
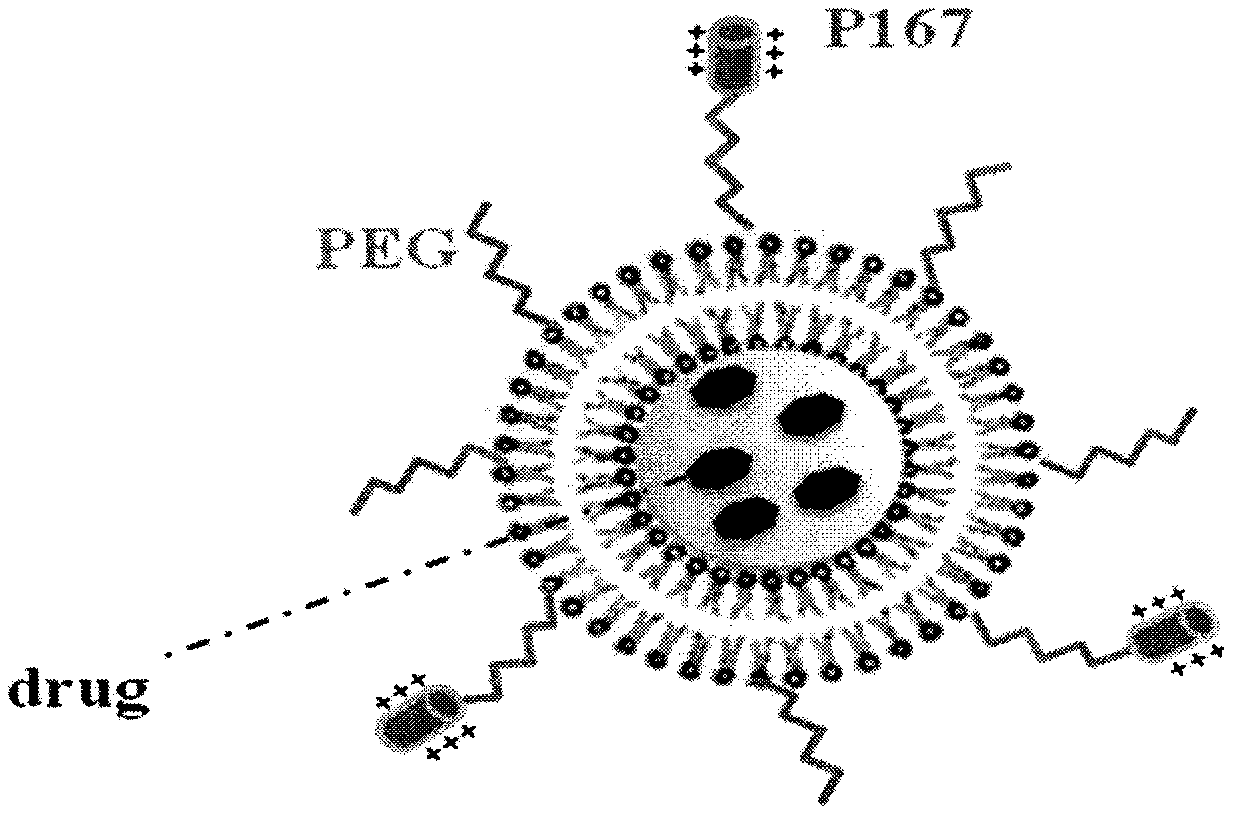
[0037] Preparation of Preliminary Example 1 Distearoylphosphatidylethanolamine-Polyethylene Glycol-P167 / P168 Complex
[0038] Weigh an appropriate amount of P167 peptide into an eggplant-shaped bottle, dissolve it with anhydrous DMF, and add triethylamine equivalent to 2.5-3 times the molar amount of the P167 / P168 peptide to adjust the pH of the solution to 7.4. Add an appropriate amount of DSPE-PEG-NHS (DSPE-PEG-NHS:P167 peptide=1.2:1, molar ratio) and react on a magnetic stirrer at room temperature for 2 days in the dark. The reaction mixture was dialyzed in 500 mL of distilled water in a dialysis bag with a molecular weight cut-off of 3500 Da for 3 days, and the dialyzed fluid was changed every 12 hours to remove incompletely reacted reactants, triethylamine and DMF. The liquid in the dialysis bag was freeze-dried at -40°C and 0.22 mbar for 24 hours to obtain a white solid powder.
experiment example 1
[0039] Experimental example 1 P167 modified Adriamycin liposome preparation process
[0040] 1. Determination of the binding mode of P167 peptide and liposome
[0041] In order to quantitatively detect the P167 peptide modification density and optimize the preparation process of P167 peptide modified liposomes with high binding rate, the P167A peptide (RRRRRRRRRK(β-Ala-FITC)-amide) with similar sequence to P167 peptide (GGRRRRRRR) was used instead. The P167A peptide introduces lysine residues on the basis of the P167 peptide, and FITC is labeled on one amino site of the lysine residues, while the other amino group can react with the activation group of PEGylated phospholipids to generate a guiding compound . In this way, the quantitative detection of the modified ligand on the liposome can be realized, which provides a research means for further investigation of the preparation process of the P167 peptide modified liposome.
[0042] Targeted compound method is a widely used ...
experiment example 2
[0059] Optimization of P167 modified density in experimental example 2 preparation
[0060] 1. The relationship between P167 peptide modification density and liposome properties
[0061] (1) Experimental method:
[0062] Adjust the dosage of P167 in the prescription in the preparation process of Example 1, and the modification densities of the P167 peptides were 0%, 1%, 2%, 4%, and 8%, respectively. Prepare doxorubicin liposome preparations without density modification by P167. The encapsulation efficiency ee% of DOX-P167-SSL preparation was determined by dextran G50 column separation method (Jiang J, Yang SJ, Wang JC, et al. Sequential treatment of drug-resistant tumors with RGD-modified liposomes containing siRNA or doxombicin[ J]. Eur J Pharm Biopharm, 2010; 76: 170-178.). The particle size of DOX-P167-SSL preparation was measured by laser light scattering instrument.
[0063] DPH is used as a fluorescent probe, the degree of fluorescence polarization p is measured by a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com