Preparation method of a gemcitabine hydrochloride freeze-dried powder injection
A technology of gemcitabine hydrochloride and freeze-dried agent, which is applied in the field of pharmaceutical preparations, can solve the problems of long time-consuming, long-time-consuming, and long production cycle of gemcitabine hydrochloride freeze-dried agent, reduce crusting and peeling, increase freeze-drying efficiency, and improve The effect of freeze-drying efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
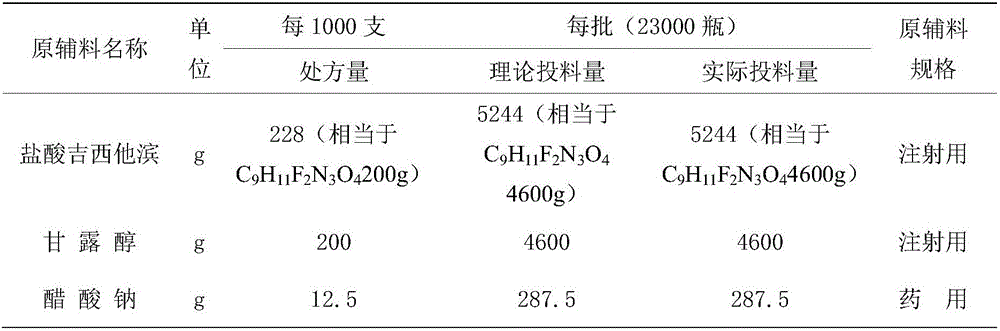
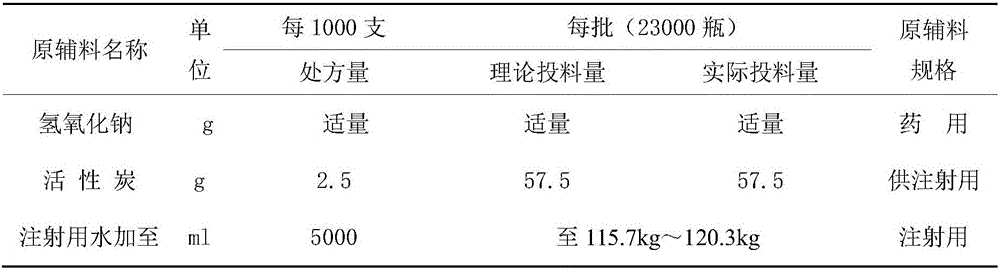
[0030] Recipe: See Table 1
[0031] Table 1: Process prescription of gemcitabine hydrochloride for injection
[0032]
[0033]
[0034] Add about 40kg of water for injection into the 50L preparation tank, add the prescribed amount of mannitol and sodium acetate into the 50L preparation tank, control the temperature of the liquid medicine at 45°C-55°C, set the stirring speed at 70%, and stir for 5 minutes to completely dissolve. After confirming that mannitol and sodium acetate are completely dissolved and that the temperature of the drug solution is within the range of 45°C to 55°C, add the prescribed amount of gemcitabine hydrochloride into a 50L preparation tank, maintain the stirring speed at 70%, and stir for 10 minutes to completely dissolve it. Use 1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 2.9-3.1. After the pH value is adjusted to pass, confirm that the temperature of the liquid medicine is within the range of 45°C to 55°C.
[0035...
Embodiment 2
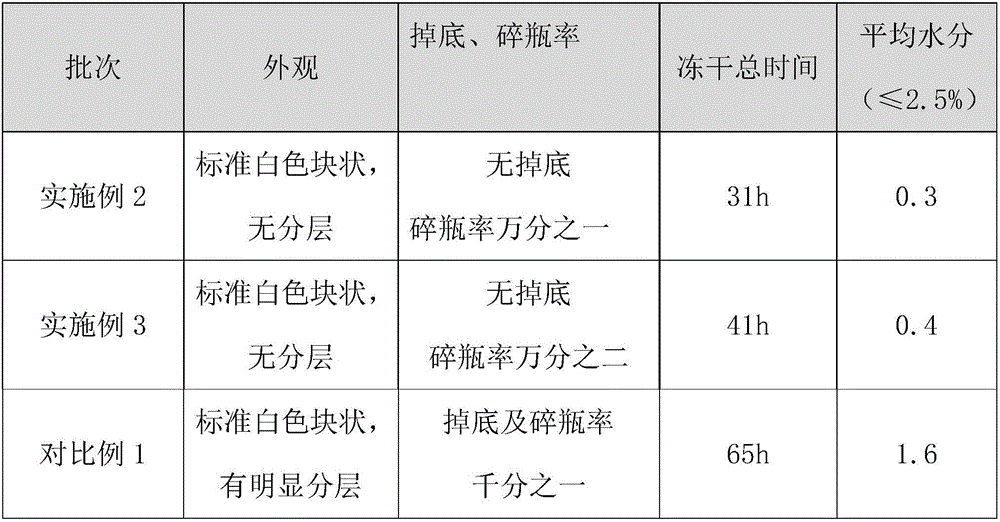
[0038] The gemcitabine hydrochloride (0.2g) solution prepared by the method of Example 1 is freeze-dried according to the following method:
[0039] a. Into the box: control the heat transfer oil inlet temperature to 20°C, and put the gemcitabine hydrochloride solution into the freeze-drying box;
[0040] b. Pre-freezing: reduce the temperature of the heat transfer oil inlet to -2°C at full speed and maintain it for 2 hours, then reduce the temperature of the heat transfer oil inlet to -45°C at full speed and maintain it for another 4 hours;
[0041] c. Condenser cooling and vacuuming: After the pre-freezing is completed, confirm that the temperature of the condenser has dropped below -45°C, and vacuum to the limit;
[0042] d. Primary drying: after the vacuum degree reaches 10Pa, it takes 240 minutes to raise the temperature of the heat transfer oil inlet from -45°C to 20°C, turn on the vacuum control, adjust the vacuum control range of the front box to 35±5Pa, observe the di...
Embodiment 3
[0046] The gemcitabine hydrochloride (1.0g) solution was freeze-dried according to the following method:
[0047] a. Into the box: control the heat transfer oil inlet temperature to 20°C, and put the gemcitabine hydrochloride solution into the freeze-drying box;
[0048] b. Pre-freezing: reduce the temperature of the heat transfer oil inlet to -2°C at full speed, and after maintaining at -2°C for 2 hours, lower the temperature of the heat transfer oil inlet to -45°C at full speed, and then maintain it at -45°C for 4 hours;
[0049] c. Condenser cooling and vacuuming: After the pre-freezing is completed, confirm that the temperature of the condenser has dropped below -45°C, and vacuum to the limit;
[0050]d. Primary drying: after the vacuum degree reaches 10Pa, it takes 240 minutes to raise the temperature of the heat transfer oil inlet from -45°C to 20°C, turn on the vacuum control, adjust the vacuum control range of the front box to 35±5Pa, observe the disappearance of the w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com