Anti-coagulation and anti-tumor polypeptide freeze-dried powder capsule and preparation method thereof
A freeze-dried powder and anti-tumor technology, which is applied in the direction of anti-tumor drugs, capsule delivery, freeze-drying delivery, etc., can solve the problems of low absorption and utilization rate, incomplete desalination, discounted nutritional value, etc., and achieve low impurities and heavy metal content, Ease of absorption and utilization, high absorption and utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
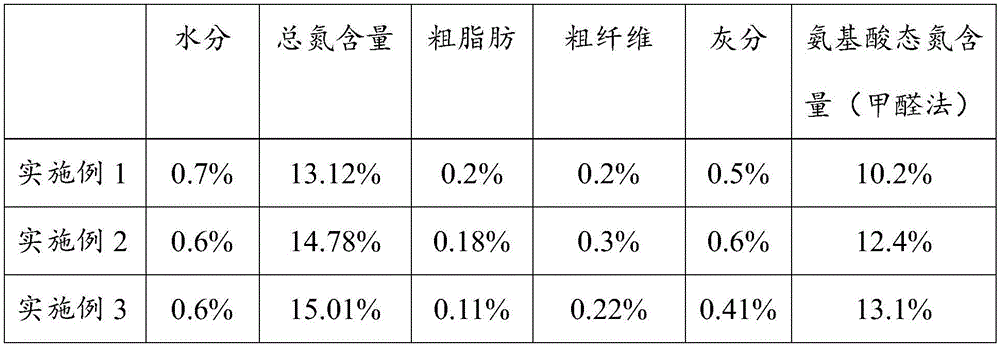
[0038] Anticoagulant and anti-tumor polypeptide freeze-dried powder capsules, the filler of the polypeptide freeze-dried powder capsule is composed of tartary buckwheat extract freeze-dried powder, sea cucumber intestinal polypeptide freeze-dried powder and bee pollen extract freeze-dried powder in a mass ratio of 10: 5:1 mixed composition;
[0039] The preparation method of the sea cucumber intestinal polypeptide freeze-dried powder comprises the following steps:
[0040] The first step is to prepare sea cucumber intestine polypeptides. (1) Sea cucumber intestine pretreatment: collect fresh sea cucumber intestines, and use pretreatment agents A and B for pretreatment. Pretreatment agent A is a water-insoluble dextran mixture with a mass fraction of 8%. Suspension, the pretreatment agent B is a reduced glutathione aqueous solution with a mass fraction of 0.5%, the specific pretreatment operation is: first use the pretreatment agent A to pretreat at room temperature for 2 hours...
Embodiment 2
[0051] Anticoagulant and anti-tumor polypeptide freeze-dried powder capsules, the filler of the polypeptide freeze-dried powder capsule is composed of tartary buckwheat extract freeze-dried powder, sea cucumber intestinal polypeptide freeze-dried powder and bee pollen extract freeze-dried powder in a mass ratio of 10: 5:1 mixed composition;
[0052] The preparation method of the sea cucumber intestinal polypeptide freeze-dried powder comprises the following steps:
[0053] The first step is to prepare sea cucumber intestine polypeptides. (1) Sea cucumber intestine pretreatment: collect fresh sea cucumber intestines, and use pretreatment agents A and B for pretreatment. Pretreatment agent A is a water-insoluble dextran mixture with a mass fraction of 12%. Suspension, the pretreatment agent B is a reduced glutathione aqueous solution with a mass fraction of 1.5%, the specific pretreatment operation is: first use the pretreatment agent A to pretreat at room temperature for 1 hour...
Embodiment 3
[0064] Anticoagulant and anti-tumor polypeptide freeze-dried powder capsules, the filler of the polypeptide freeze-dried powder capsule is composed of tartary buckwheat extract freeze-dried powder, sea cucumber intestinal polypeptide freeze-dried powder and bee pollen extract freeze-dried powder in a mass ratio of 10: 5:1 mixed composition;
[0065] The preparation method of the sea cucumber intestinal polypeptide freeze-dried powder comprises the following steps:
[0066] The first step is to prepare sea cucumber intestine polypeptides. (1) Sea cucumber intestine pretreatment: collect fresh sea cucumber intestines, and use pretreatment agents A and B for pretreatment. Pretreatment agent A is a water-insoluble dextran mixture with a mass fraction of 10%. suspension, the pretreatment agent B is a reduced glutathione aqueous solution with a mass fraction of 1.0%, and the specific pretreatment operation is: mix the pretreatment agent A and the pretreatment agent B uniformly at a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mwco | aaaaa | aaaaa |
| Mwco | aaaaa | aaaaa |
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com