A kind of comprehensive utilization method of ketone alcohol mixture
A mixture, cyclohexanone technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of high cost, complex operation, high energy consumption, achieve good hydrogenation effect, no safety risk. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
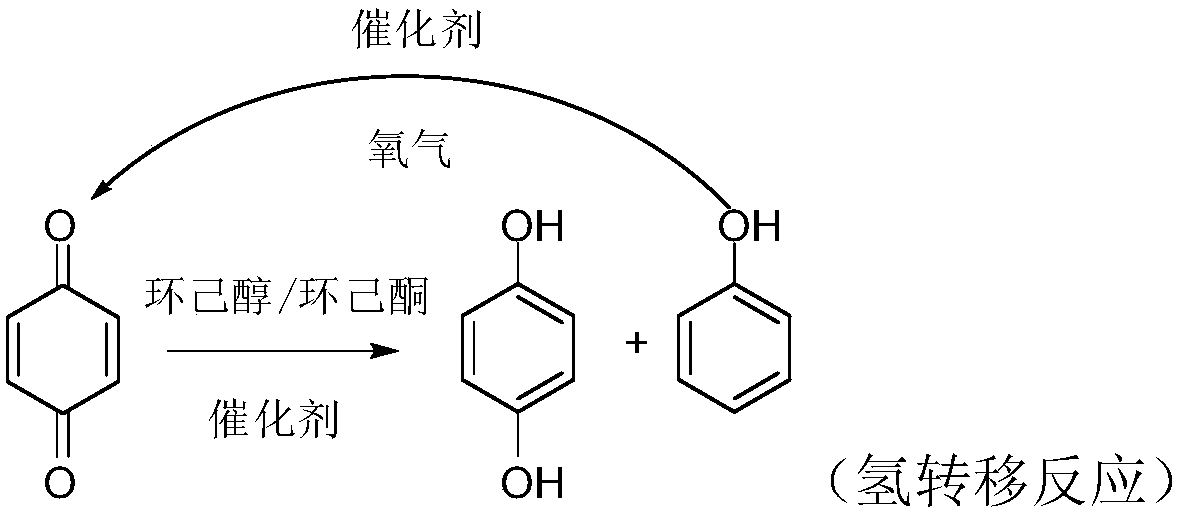
[0020] Weigh 10.8g p-benzoquinone, 30g cyclohexanol / cyclohexanone mixture (80wt% cyclohexanone content) and 0.32g5wt% platinum cobalt cerium supported titanium dioxide catalyst (platinum: cobalt: cerium mass ratio is 72:18:10) Put it into a 500ml flask, keep it under nitrogen protection at 120°C for 8 hours, cool down to room temperature after the reaction, the catalyst sinks at the bottom of the flask, pour out the reaction solution and filter it with a Buchner funnel to separate the catalyst, and the obtained filtrate is distilled under reduced pressure 25 g of unreacted cyclohexanol / cyclohexanone mixture, 4.2 g of phenol and 9.9 g of hydroquinone were separated off in succession. The conversion rate of cyclohexanone is 20.1%, and the yield of hydroquinone is 90% based on p-benzoquinone. 4.2g of phenol, 70g of ethanol and 0.2g of copper chloride were reacted at 80°C and 2.5MPa of oxygen for 3 hours. After the reaction, about 10g of benzenetrifluoromethane was added, stirred ...
Embodiment 2
[0022] Take by weighing 10.8g p-benzoquinone, 30g cyclohexanol / cyclohexanone mixture (cyclohexanone content 65wt%) and 0.28g6wt% palladium copper lanthanum supported activated carbon catalyst (palladium: copper: lanthanum mass ratio is 71:18:11) Put it into a 500ml flask, and keep it under the protection of nitrogen for 7 hours at 130°C. After the reaction, it is cooled to room temperature. The catalyst sinks at the bottom of the flask. Pour out the reaction solution and filter it with a Buchner funnel to separate the catalyst. 24 g of unreacted cyclohexanol / cyclohexanone mixture, 4.1 g of phenol and 9.6 g of hydroquinone were separated off in succession. The conversion rate of cyclohexanone is 23.4%, and the yield of hydroquinone is 87.3% based on p-benzoquinone. 4.1g of phenol, 70g of ethanol and 0.2g of copper chloride were reacted at 80°C and 2.5MPa of oxygen for 3 hours. After the reaction, about 12g of benzenetrifluoromethane was added, stirred for 30 minutes, and left s...
Embodiment 3
[0024] Take by weighing 10.6g p-benzoquinone, 35g cyclohexanol / cyclohexanone mixture (cyclohexanone content 50wt%) and 0.3g5wt% rhodium nickel lanthanum supported activated carbon catalyst (rhodium: nickel: lanthanum mass ratio is 73:17:10) Put it into a 500ml flask, under the protection of nitrogen, keep warm at 125°C for 6.5h. After the reaction is completed, the temperature is lowered to room temperature. The catalyst sinks to the bottom of the flask. Distillation successively separated off 28 g of unreacted cyclohexanol / cyclohexanone mixture, 4.2 g of phenol and 9.8 g of hydroquinone. The conversion rate of cyclohexanone is 27.6%, and the yield of hydroquinone is 89.1% based on p-benzoquinone. 4.2g of phenol, 70g of ethanol and 0.2g of copper chloride were reacted at 80°C and 2.5MPa of oxygen for 3 hours. After the reaction, about 15g of benzenetrifluoromethane was added, stirred for 30 minutes, and left standing overnight, p-benzoquinone was precipitated in the form of pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com