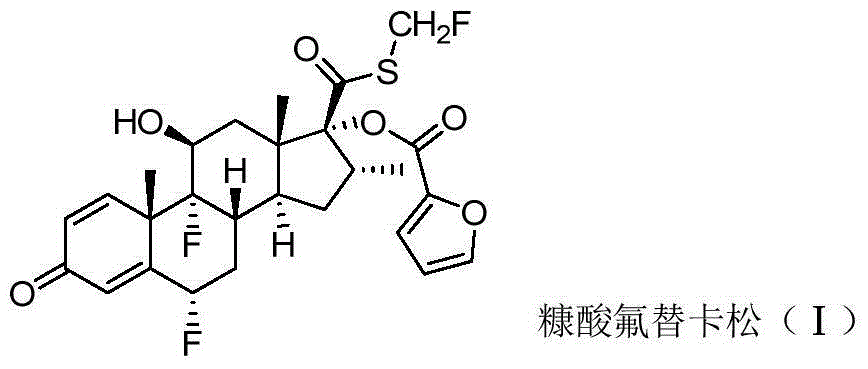
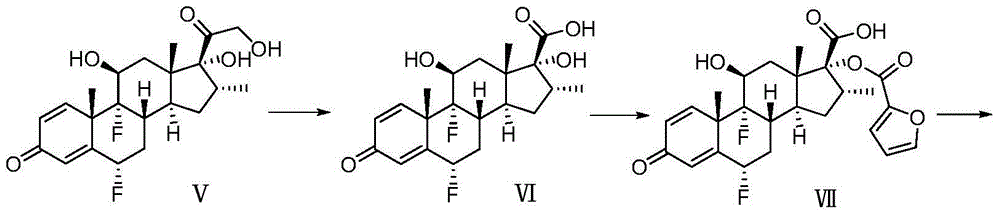
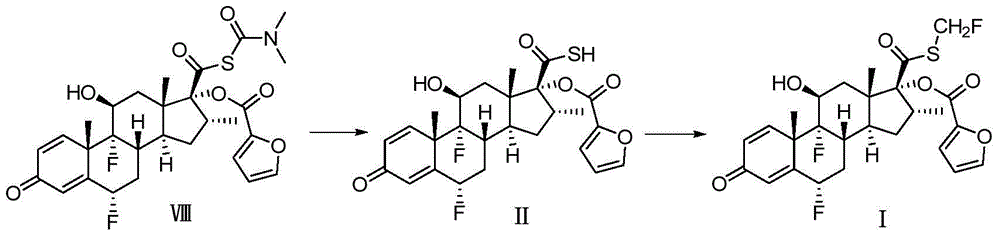
Preparation method of fluticasone furoate
An acid regulation and compound technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of increasing the difficulty of refining, affecting the yield, and difficult to purify, and achieving the effect of reducing the difficulty of refining, ensuring safety, and reducing residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 6α,9α-Difluoro-17α-[(2-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl-3-oxo-androst-1,4-diene-17β-thiocarboxy Preparation of Acid-S-N,N-Dimethylcarbamoyl Ester
[0040]
[0041] Add 2-butanone (3.33L) into a 10L round-bottomed glass flask, add compound VII (222g, 0.453mol) under stirring, control the temperature at 10-20°C and stir evenly, then add triethylamine (190mL, 1.359mol) dropwise ); after 10 min, N,N-dimethylthiocarbamoyl chloride (140 g, 1.133 mol) was added, and after another 10 min, an aqueous solution of sodium iodide (81.5 g, 0.544 mol) was added; stirred at 15°C to 20°C for 5h, TLC Monitor until the reaction is complete. Add N,N-dimethylacetamide (1.78L) to the reaction solution, stir for 10 minutes, then add dropwise purified water pre-cooled to below 5°C, stir for 1.0h at 0°C to 10°C after dropping, filter with suction, use for filter cake After washing with purified water and suction filtration, the obtained wet product was air-dried at 45°C±3°C for 12...
Embodiment 2
[0043] 6α,9α-Difluoro-17α-[(2-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl-3-oxo-androst-1,4-diene-17β-thiocarboxy acid preparation
[0044]
[0045] Anhydrous methanol (2.195L) was added to a 3L round bottom glass flask, and intermediate VIII (219.5g, 0.38mol) and potassium carbonate (157.6g, 1.14mol) were added successively, N 2 Under protection, control the temperature at 40-45° C. and stir for 4.0 h, and monitor by TLC until the reaction is complete. In a 10L three-necked flask, add the reaction solution to purified water pre-cooled to 0-5°C, and slowly add 2mol / L hydrochloric acid (0.222L hydrochloric acid / 1.11L water) at 0-10°C under temperature control. Stir for 1.0 h, filter with suction, wash the solid with purified water, and filter with suction, the obtained crude product is analyzed by HPLC, the purity is ≥96%, and the proportions of impurities IX and VIII are greater than 1.2% and 1.0%, respectively.
[0046] Add the crude product to Na with stirring 2 CO 3 (5...
Embodiment 3
[0048] 6α,9α-Difluoro-17α-[(2-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl-3-oxo-androst-1,4-diene-17β-thiocarboxy acid preparation
[0049]
[0050] Intermediate VIII (1.00g, 1.73mmol) and potassium phosphate (1.10g, 5.20mmol) were dissolved in 10mL of anhydrous methanol; N 2 Under protection, control the temperature at 40-45°C and stir for 4.0 hours until the reaction of intermediate VIII is completed; add the reaction solution to 25 mL of pre-cooled purified water, add 8.6 mL of 2 mol / L hydrochloric acid solution dropwise under stirring; filter with suction and wash with water to obtain the intermediate Ⅱ crude product. Add the crude product to a mixed solution of sodium carbonate (0.5g) / purified water (11mL), add 4mL of ethyl acetate and stir to dissolve it, then separate the liquids, then stir and wash the water phase with 4mL of ethyl acetate, and add the water phase 8.6 mL of 2 mol / L hydrochloric acid solution was suction filtered, washed with water, and dried under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com