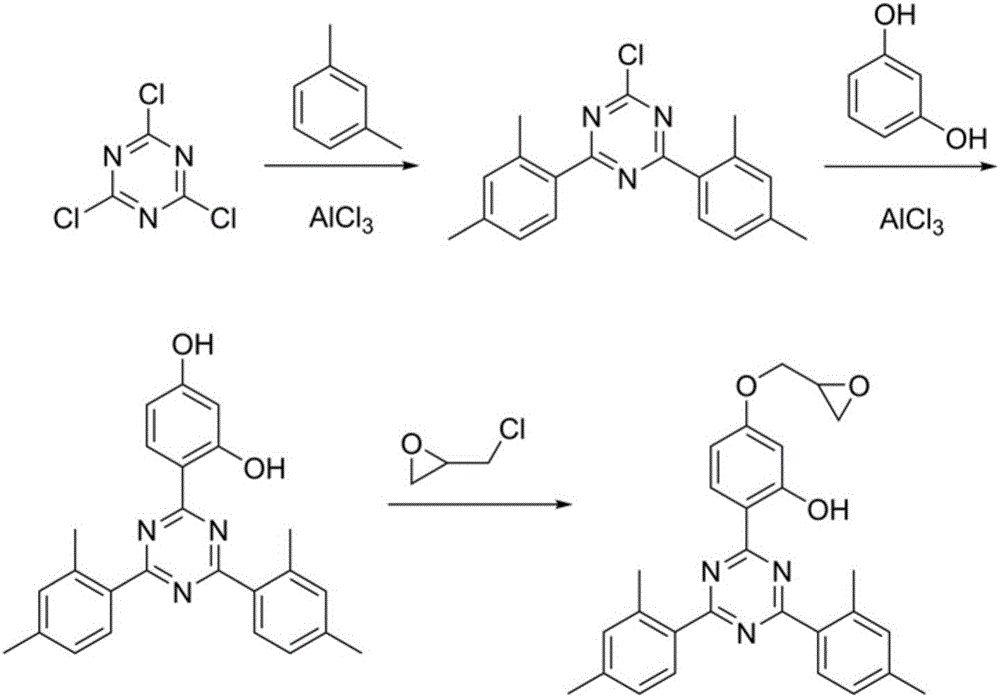
Method for synthesizing 2-(4,6-di(2,4-xylyl)-1,3,5-triazine-2-base)-5-glycidyl ether phenol
A glycidyl ether and xylylene technology, which is applied in the field of organic synthesis, can solve the problems of increasing production costs and waste treatment costs, high boiling point, and increasing the discharge of harmful substances, and saves production costs, waste treatment costs and product yield. High, responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
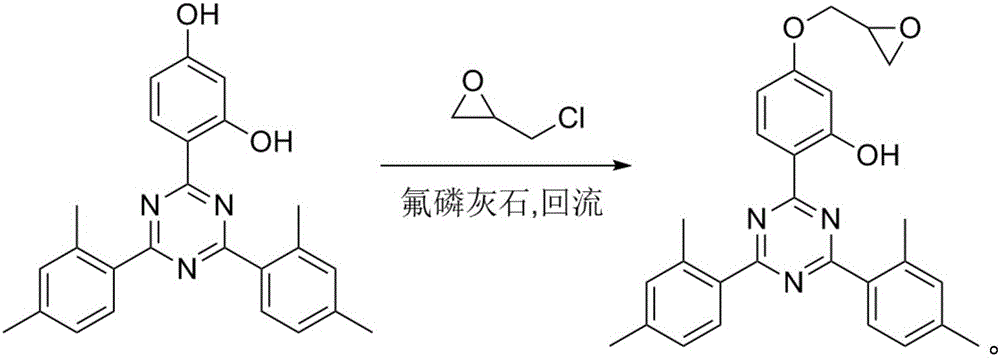
[0027] Example 1 20% Fluorapatite and a reaction temperature of 100°C (chloroform dissolves the reaction product)
[0028] 5 grams of 4-[4,6-di(2,4-xylyl)-1,3,5-triazin-2-yl]-1,3-resorcinol, 1 gram of fluorapatite 1. Add 50 milliliters of epichlorohydrin to a 100 milliliter three-necked flask, install a reflux condenser and a thermometer. Heat to 100°C and heat at this temperature for 12 hours, complete consumption of starting material is followed by TLC. Cool to room temperature and filter. The resulting solid was dissolved in 5 ml of chloroform and filtered to remove fluorapatite. The resulting filtrate was concentrated to dryness and dried in vacuo to obtain 5.25 g of crude product (yield 92%).
Embodiment 2
[0029] Example 2 20% fluorapatite and a reaction temperature of 118°C (chloroform dissolves the reaction product)
[0030] 5 grams of 4-[4,6-di(2,4-xylyl)-1,3,5-triazin-2-yl]-1,3-resorcinol, 1 gram of fluorapatite 1. Add 50 milliliters of epichlorohydrin into a 100 milliliter three-necked flask, and install a reflux condenser. Heat to reflux (the boiling point of epichlorohydrin is 117.9° C.), heat at this temperature for 7 hours, and use thin layer chromatography to track that the starting material is consumed. Cool to room temperature and filter. The resulting solid was dissolved in 5 ml of chloroform and filtered to remove fluorapatite. The resulting filtrate was concentrated to dryness and dried in vacuo to obtain 5.14 g of crude product (yield 90%).
Embodiment 3
[0031] Example 3 15% fluorapatite and a reaction temperature of 118°C (chloroform dissolves the reaction product)
[0032] 5 grams of 4-[4,6-bis(2,4-xylyl)-1,3,5-triazin-2-yl]-1,3-resorcinol, 0.75 grams of fluorapatite 1. Add 50 milliliters of epichlorohydrin into a 100 milliliter three-necked flask, and install a reflux condenser. Heat to reflux (the boiling point of epichlorohydrin is 117.9° C.), heat at this temperature for 7 hours, and use thin layer chromatography to track that the starting material is consumed. Cool to room temperature and filter. The resulting solid was dissolved in 5 ml of chloroform and filtered to remove fluorapatite. The resulting filtrate was concentrated to dryness and dried in vacuo to obtain 5.20 g of crude product (yield 91%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com